Functional Interaction between the Scaffold Protein Kidins220/ARMS and Neuronal Voltage-Gated Na+ Channels - PubMed (original) (raw)
Functional Interaction between the Scaffold Protein Kidins220/ARMS and Neuronal Voltage-Gated Na+ Channels
Fabrizia Cesca et al. J Biol Chem. 2015.
Abstract
Kidins220 (kinase D-interacting substrate of 220 kDa)/ankyrin repeat-rich membrane spanning (ARMS) acts as a signaling platform at the plasma membrane and is implicated in a multitude of neuronal functions, including the control of neuronal activity. Here, we used the Kidins220(-/-) mouse model to study the effects of Kidins220 ablation on neuronal excitability. Multielectrode array recordings showed reduced evoked spiking activity in Kidins220(-/-) hippocampal networks, which was compatible with the increased excitability of GABAergic neurons determined by current-clamp recordings. Spike waveform analysis further indicated an increased sodium conductance in this neuronal subpopulation. Kidins220 association with brain voltage-gated sodium channels was shown by co-immunoprecipitation experiments and Na(+) current recordings in transfected HEK293 cells, which revealed dramatic alterations of kinetics and voltage dependence. Finally, an in silico interneuronal model incorporating the Kidins220-induced Na(+) current alterations reproduced the firing phenotype observed in Kidins220(-/-) neurons. These results identify Kidins220 as a novel modulator of Nav channel activity, broadening our understanding of the molecular mechanisms regulating network excitability.
Keywords: gating; hippocampus; neuron; patch clamp; scaffold protein; sodium channel.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
FIGURE 1.
Reduced evoked spiking activity in Kidins220−/− hippocampal networks. A, Raster plots of spontaneous spiking activity recorded from wild-type (WT) and Kidins220−/− hippocampal cultures at 21 days in vitro, shown for individual microelectrodes of the MEA device (vertical axis) and a 75-s time window (horizontal axis). B, mean firing rate of WT (n = 8) and Kidins220−/− (n = 16) cultures averaged over a 5-min recording period. C, Raster plots of evoked spiking activity recorded from WT and Kidins220−/− hippocampal cultures, shown for a 150-ms time window after stimulus delivery (horizontal axis). D, post-stimulus time histogram of evoked spiking activity averaged over 50 consecutive stimulations delivered at 0.1 Hz. Bin size was 5 ms. Number of cultures as in B.
FIGURE 2.
Kidins220 ablation alters the firing properties of hippocampal neurons. A, typical current-clamp recordings of action potential firing evoked by somatic current injection in autaptic hippocampal neurons from wild-type (WT; black traces) and Kidins220−/− mouse embryos (colored traces). Holding potential was −70 mV. Recordings were made in the subpopulations of inhibitory neurons (_A_1, Kidins220−/− in blue) and excitatory neurons (_A_2, Kidins220−/− in green). Scale bars: 200 ms/40 mV. B, mean threshold current amplitudes in inhibitory neurons (_B_1; n = 24 for WT; n = 23 for Kidins220−/−) and excitatory neurons (_B_2; n = 24 for WT; n = 21 for Kidins220−/−). C, plot of the mean instantaneous firing frequency (i-F) versus injected current amplitude in inhibitory neurons (_C_1) and excitatory neurons (_C_2). D, representative shapes of the first action potential evoked by current injection (20-pA steps) in an inhibitory (_D_1) and an excitatory neuron (_D_2). Scale bars, 10 ms/20 mV. E, mean action potential width at half-maximal amplitude in inhibitory (_E_1) and excitatory neurons (_E_2). F, phase-plane plots of the first action potential evoked by current injection (20-pA steps) in an inhibitory (_F_1) and an excitatory neuron (_F_2). G, summary plot of the mean maximum rising slope (dV/dt) and mean peak potential (_V_peak), derived from phase-plane plots as shown in F, for inhibitory (_G_1) and excitatory neurons (_G_2). C, E, and G, number of cells as in B.
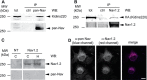
FIGURE 3.
Interaction between Kidins220 and Nav channels. A, endogenous Kidins220 and Nav channels associate in the adult mouse brain. Brain cortices were dissected from adult wild-type (C57BL6/J) mice, lysed, and immunoprecipitated by using anti-pan-Nav antibodies, or anti-GFP antibodies as control (ctrl). Samples were analyzed by SDS-PAGE and immunoblotting with anti-Kidins220 and anti-pan-Nav antibodies, as indicated. B, exogenously expressed Kidins220 and Nav channels associate in HEK293 cells. HEK293 cells were co-transfected with plasmids coding for HA-Kidins220 and Nav1.2, lysed, and immunoprecipitated with anti-Nav1.2 antibodies, or anti-GFP antibodies as control (ctrl). Samples were analyzed by SDS-PAGE and immunoblotting with anti-HA and anti-Nav1.2 antibodies, as indicated. tot, 50 μg of total cortex or cell lysate. C, COS cells (C) and HEK293 cells (H) were transiently transfected with Nav1.2, lysed, and processed by SDS-PAGE. Nitrocellulose membranes were probed with anti-Nav1.2 and anti-pan-Nav antibodies, as indicated. Lysate from non-transfected cells (NT, 50 μg) was run as a control. D, HEK293 cells were transiently transfected with Nav1.2, fixed after 48 h, and processed for immunocytochemistry with the indicated antibodies. Scale bar, 10 μm. WB, Western blot.
FIGURE 4.
Kidins220 co-expression slows the activation and inactivation kinetics of Nav1.2-mediated Na+ currents in HEK293 cells. A, whole cell current recordings on HEK293 cells transiently transfected with Nav1.2 alone (black traces) or co-transfected with Nav1.2 and Kidins220 (red traces). Currents were elicited by step depolarization of 20-ms duration from −60 to +70 mV in 10-mV increments, from a holding potential of −100 mV. Inset, superimposition of current recordings at −20 mV, normalized to the peak current amplitude. B, summary plot of the fast and slow time constants of Na+ current inactivation for HEK293 cells expressing Nav1.2 alone (gray symbols; n = 24) and for cells co-expressing Nav1.2 and Kidins220 (red symbols; n = 46). Time constants were determined by fitting the current decay at −20 mV (upon step depolarization for 150 ms) with a double-exponential function. Dashed lines indicate the mean ± 2 S.D. of the fast and slow time constants, respectively, for control cells expressing Nav1.2 alone (mean values shown as filled circle). The dotted circle encompasses those Kidins220 co-expressing cells showing slow inactivation kinetics (si-Kids). C, occurrence of si-Kids cells (see B) among all cells co-expressing Nav1.2 and Kidins220 plotted as a function of the Kidins220:Nav1.2 cDNA ratio used for HEK293 transfection. The absolute number of si-Kids cells and the total number of cells tested in each condition are indicated above each bar (si-Kids/total). D–G, the fast time constant of inactivation (D), the slow time constant of inactivation (E), the relative amplitudes of the two components of current decay (F), and the time to peak current (G) are plotted as a function of the membrane potential. In F, the relative amplitude of the fast time constant is indicated by circles (as in D), the relative amplitude of the slow time constant is indicated by squares (as in E). Data points represent mean ± S.E. of 24 control cells, 12 si-Kids, and 34 fi-Kids (Kidins220 co-expressing cells showing fast inactivation kinetics). The number symbol (#) in F indicates that si-Kids values were significantly different at all membrane potentials, with Student's t test significance levels ranging from * to ***. Values of fi-Kids cells in D–G were not significantly different from control cells (for reasons of clarity only shown for V = −20 mV).
FIGURE 5.
Kidins220 co-expression alters the voltage-dependent activation and fast inactivation of Nav1.2-mediated Na+ currents in HEK293 cells. A, current-voltage relationships recorded in HEK293 cells transfected with Nav1.2 alone (control cells; n = 24; black symbols) or co-transfected with Nav1.2 and Kidins220 (si-Kids cells; n = 12; red symbols). Peak amplitudes were normalized to the cell capacitance to obtain current densities. B, voltage dependence of Nav channel activation derived from current-voltage relationships, as described under ”Experimental Procedures“. Symbols and n values are as described in A. Smooth lines represent Boltzmann functions using the mean values for Va and ka determined from fits of single experiments (Table 2). C, voltage dependence of fast inactivation assessed in response to 150-ms inactivating pre-pulses. Symbols and n values are as described in A. Smooth lines represent Boltzmann functions using the mean values for Vi and ki determined from fits of single experiments (Table 2). D, time-dependent recovery from fast inactivation at recovery test potentials of −140, −120, and −100 mV. Symbols as in A, n = 21–23 for control cells, n = 8–10 for si-Kids cells. Smooth lines represent fits to a monoexponential function. E, the time constant of recovery from fast inactivation, determined from data fits in D, is plotted as a function of the test potential during the recovery period. Symbols as in A, n values as in D. F and G, computational modeling of interneuronal excitability. In simulated Kidins220−/− neurons, the Nav channel activation and inactivation curves were shifted toward negative potentials by 10 mV (for WT: Va = −20 mV, Vi = −40 mV; for Kidins220−/−: Va = −30 mV, Vi = −50 mV). F, maximum rising slope of action potentials derived from the simulation (sim) and experimental data (exp; Fig. 2_G_1). G, plot of the instantaneous firing frequency (i-F) versus injected current amplitude in simulated WT (black line) and Kidins220−/− neurons (blue line). The dashed line corresponds to simulated Kidins220−/− neurons with the additional constraint of faster inactivation kinetics.
Similar articles
- Stepping Out of the Shade: Control of Neuronal Activity by the Scaffold Protein Kidins220/ARMS.
Scholz-Starke J, Cesca F. Scholz-Starke J, et al. Front Cell Neurosci. 2016 Mar 14;10:68. doi: 10.3389/fncel.2016.00068. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27013979 Free PMC article. Review. - Kidins220/ARMS is a novel modulator of short-term synaptic plasticity in hippocampal GABAergic neurons.
Scholz-Starke J, Cesca F, Schiavo G, Benfenati F, Baldelli P. Scholz-Starke J, et al. PLoS One. 2012;7(4):e35785. doi: 10.1371/journal.pone.0035785. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563401 Free PMC article. - Ankyrin repeat-rich membrane spanning/Kidins220 protein interacts with mammalian Septin 5.
Park HJ, Park HW, Lee SJ, Arevalo JC, Park YS, Lee SP, Paik KS, Chao MV, Chang MS. Park HJ, et al. Mol Cells. 2010 Aug;30(2):143-8. doi: 10.1007/s10059-010-0099-7. Epub 2010 Jul 23. Mol Cells. 2010. PMID: 20680483 - Developmental and activity-dependent regulation of ARMS/Kidins220 in cultured rat hippocampal neurons.
Cortés RY, Arévalo JC, Magby JP, Chao MV, Plummer MR. Cortés RY, et al. Dev Neurobiol. 2007 Nov;67(13):1687-98. doi: 10.1002/dneu.20542. Dev Neurobiol. 2007. PMID: 17587220 - Kidins220 and tumour development: Insights into a complexity of cross-talk among signalling pathways (Review).
Cai S, Cai J, Jiang WG, Ye L. Cai S, et al. Int J Mol Med. 2017 Oct;40(4):965-971. doi: 10.3892/ijmm.2017.3093. Epub 2017 Aug 9. Int J Mol Med. 2017. PMID: 28849114 Free PMC article. Review.
Cited by
- Ankyrin-rich membrane spanning protein as a novel modulator of transient receptor potential vanilloid 1-function in nociceptive neurons.
Peter J, Kasper C, Kaufholz M, Buschow R, Isensee J, Hucho T, Herberg FW, Schwede F, Stein C, Jordt SE, Brackmann M, Spahn V. Peter J, et al. Eur J Pain. 2017 Jul;21(6):1072-1086. doi: 10.1002/ejp.1008. Epub 2017 Feb 9. Eur J Pain. 2017. PMID: 28182310 Free PMC article. - Transient Receptor Potential Vanilloid 1 Signaling Is Independent on Protein Kinase A Phosphorylation of Ankyrin-Rich Membrane Spanning Protein.
Pellegrino A, Mükusch S, Seitz V, Stein C, Herberg FW, Seitz H. Pellegrino A, et al. Med Sci (Basel). 2022 Nov 17;10(4):63. doi: 10.3390/medsci10040063. Med Sci (Basel). 2022. PMID: 36412904 Free PMC article. - Nav1.7-A1632G Mutation from a Family with Inherited Erythromelalgia: Enhanced Firing of Dorsal Root Ganglia Neurons Evoked by Thermal Stimuli.
Yang Y, Huang J, Mis MA, Estacion M, Macala L, Shah P, Schulman BR, Horton DB, Dib-Hajj SD, Waxman SG. Yang Y, et al. J Neurosci. 2016 Jul 13;36(28):7511-22. doi: 10.1523/JNEUROSCI.0462-16.2016. J Neurosci. 2016. PMID: 27413160 Free PMC article. - Stepping Out of the Shade: Control of Neuronal Activity by the Scaffold Protein Kidins220/ARMS.
Scholz-Starke J, Cesca F. Scholz-Starke J, et al. Front Cell Neurosci. 2016 Mar 14;10:68. doi: 10.3389/fncel.2016.00068. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27013979 Free PMC article. Review. - Kidins220/ARMS modulates brain morphology and anxiety-like traits in adult mice.
Almacellas-Barbanoj A, Albini M, Satapathy A, Jaudon F, Michetti C, Krawczun-Rygmaczewska A, Huang H, Manago F, Papaleo F, Benfenati F, Cesca F. Almacellas-Barbanoj A, et al. Cell Death Discov. 2022 Feb 9;8(1):58. doi: 10.1038/s41420-022-00854-4. Cell Death Discov. 2022. PMID: 35140204 Free PMC article.
References
- Iglesias T., Cabrera-Poch N., Mitchell M. P., Naven T. J., Rozengurt E., Schiavo G. (2000) Identification and cloning of Kidins220, a novel neuronal substrate of protein kinase D. J. Biol. Chem. 275, 40048–40056 - PubMed
- Neubrand V. E., Cesca F., Benfenati F., Schiavo G. (2012) Kidins220/ARMS as a functional mediator of multiple receptor signalling pathways. J. Cell Sci. 125, 1845–1854 - PubMed
- Cortés R. Y., Arévalo J. C., Magby J. P., Chao M. V., Plummer M. R. (2007) Developmental and activity-dependent regulation of ARMS/Kidins220 in cultured rat hippocampal neurons. Dev. Neurobiol. 67, 1687–1698 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases