A Noncanonical Autophagy Pathway Restricts Toxoplasma gondii Growth in a Strain-Specific Manner in IFN-γ-Activated Human Cells - PubMed (original) (raw)
A Noncanonical Autophagy Pathway Restricts Toxoplasma gondii Growth in a Strain-Specific Manner in IFN-γ-Activated Human Cells
Elizabeth M Selleck et al. mBio. 2015.
Abstract
A core set of autophagy proteins is required for gamma interferon (IFN-γ)-mediated clearance of Toxoplasma gondii in the mouse because of their control of several downstream effectors, including immunity-related GTPases (IRGs) and guanylate-binding proteins (GBPs). However, these effectors are absent (i.e., IRGs) from or nonessential (i.e., GBPs) in IFN-γ-activated human cells, raising the question of how these cells control parasite replication. Here, we define a novel role for ubiquitination and recruitment of autophagy adaptors in the strain-specific control of T. gondii replication in IFN-γ-activated human cells. Vacuoles containing susceptible strains of T. gondii became ubiquitinated, recruited the adaptors p62 and NDP52, and were decorated with LC3. Parasites within LC3-positive vacuoles became enclosed in multiple layers of host membranes, resulting in stunting of parasite replication. However, LC3-positive T. gondii-containing vacuoles did not fuse with endosomes and lysosomes, indicating that this process is fundamentally different from xenophagy, a form of autophagy involved in the control of intracellular bacterial pathogens. Genetic knockout of ATG16L or ATG7 reverted the membrane encapsulation and restored parasite replication, indicating that core autophagy proteins involved in LC3 conjugation are important in the control of parasite growth. Despite a role for the core autophagy machinery in this process, upstream activation through Beclin 1 was not sufficient to enhance the ubiquitination of T. gondii-containing vacuoles, suggesting a lack of reliance on canonical autophagy. These findings demonstrate a new mechanism for IFN-γ-dependent control of T. gondii in human cells that depends on ubiquitination and core autophagy proteins that mediate membrane engulfment and restricted growth.
Importance: Autophagy is a process of cellular remodeling that allows the cell to recycle senescent organelles and recapture nutrients. During innate immune responses in the mouse, autophagy is recruited to help target intracellular pathogens and thus eliminate them. However, the antimicrobial mediators that depend on autophagy in the mouse are not conserved in humans, raising the issue of how human cells control intracellular pathogens. Our study defines a new pathway for the control of the ubiquitous intracellular parasite T. gondii in human cells activated by IFN-γ. Recruitment of autophagy adaptors resulted in engulfment of the parasite in multiple membranes and growth impairment. Although susceptible type 2 and 3 stains of T. gondii were captured by this autophagy-dependent pathway, type 1 strains were able to avoid entrapment.
Copyright © 2015 Selleck et al.
Figures
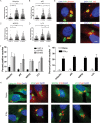
FIG 1
Recruitment of autophagy adaptors to intracellular T. gondii. (A to D) Quantification of recruitment of autophagy adaptors to type 1 (GT-1), 2 (PTG), or 3 (VEG) T. gondii in HeLa cells activated for 24 h with IFN-γ prior to infection and analyzed at 6 h postinfection. Data points represent the mean red fluorescence of the respective host markers in a ROI overlapping the T. gondii vacuole. Arrowheads indicate the mean red fluorescence value of 90% of visually determined positive vacuoles. Each value is the mean ± SEM of three experiments (***, P ≤ 0.001; *, P ≤ 0.05; Kruskal-Wallis test). (E) Immunofluorescence localization of ubiquitin, p62, NDP52, or LC3 to the PVM in HeLa cells activated with 5 ng/ml IFN-γ at 6 h postinfection with type 3 (VEG) parasites. Ubiquitin was localized with mouse MAb FK2, followed by anti-mouse IgG conjugated to Alexa Fluor 594 (red). p62 was localized with a guinea pig polyclonal antibody, followed by anti-guinea pig IgG conjugated to Alexa Fluor 594 (red). NDP52 was localized with a rabbit polyclonal antibody, followed by anti-rabbit IgG conjugated to Alexa Fluor 594 (red). LC3 was localized with a rabbit polyclonal antibody, followed by Alexa Fluor 594 (red). T. gondii PVM was localized with either a rabbit polyclonal antibody to GRA7, followed anti-rabbit IgG conjugated to Alexa Fluor 488 (green), or mouse MAb tg17-113 to GRA5, followed by anti-mouse IgG conjugated to Alexa Fluor 488 (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar, 5 µm. (F) Visual assessment of the percentage of host marker-positive T. gondii PVs from images used to collect the data shown in panels A to D. Each value is the mean ± SEM of three experiments. (G) Quantification of recruitment of autophagy proteins to type 3 (VEG) parasites in naive or IFN-γ-activated HeLa cells at 6 h postinfection by fluorescence microscopy. There were two experiments and a total of six coverslips. Each value is the mean ± SD. (H) Immunofluorescence images of HeLa cells infected with type 3 (VEG) parasites at 6 h postinfection. HeLa cells were activated with 5 ng/ml IFN-γ. Ubiquitin was localized with mouse MAb FK2, p62 was localized with a guinea pig polyclonal antibody, NDP52 was localized with a rabbit polyclonal antibody, and LC3 was localized with a rabbit polyclonal antibody, followed by secondary antibodies conjugated to Alexa Fluor 488 (green) or 594 (red), as indicated. T. gondii PVM was localized with either a rabbit polyclonal antibody to GRA7 or mouse MAb tg17-113 to GRA5, with the opposite antibody species to the host marker, followed by anti-mouse IgG conjugated to Alexa Fluor 488 (green) or 549 (red). Nuclei were stained with DAPI. Scale bar, 5 µm. See also Fig. S1 in the supplemental material.
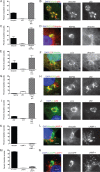
FIG 2
Colocalization of autophagy adaptors in intracellular T. gondii. (A, C, E, G, I, K, M) Quantification of colocalization of host cell markers. The percentage of the subset of type 3 (VEG) PVs that were positive for either a single host cell marker or both was determined by microscopic examination in HeLa cells or in HeLa cells expressing LC3-GFP (M) activated with 5 ng/ml IFN-γ. At 6 h postinfection, cells were fixed and stained for immunofluorescence localization of host cell markers as described below. Each value is the mean ± SEM of three experiments. (K) Mean values ± SD of two experiments with three internal replicates each. (B, D, F, H, J, L, N) Representative images of colocalization of autophagy adaptors surrounding T. gondii. Ubiquitin was localized with mouse MAb FK2, p62 was localized with a guinea pig polyclonal antibody, NDP52 was localized with a rabbit polyclonal antibody, LC3 was localized with a rabbit polyclonal antibody, LAMP-1 was localized with a rabbit polyclonal antibody, and GFP-LC3 was localized with mouse MAb 3E6 to GFP, followed by secondary antibodies conjugated with either Alexa Fluor 488 (green) or 594 (red), as indicated. Nuclei were stained with DAPI. Scale bars, 5 µm. See also Fig. S2 in the supplemental material.
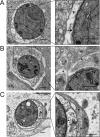
FIG 3
Engulfment of T. gondii (Tg) by multiple membranes in IFN-γ-activated cells. (A) Transmission EM reveals the normal architecture of the PVM of a type 3 (VEG) parasite in a naive HeLa cell at 6 h postinfection. (B, C) Additional membranes surround the PVM of a type 3 (VEG) parasite in HeLa cells activated with 5 ng/ml IFN-γ at 6 h postinfection. The arrows indicate the parasite membrane, the arrowheads indicate the PVM, and the asterisks indicate additional membranes. Scale bars, 500 nm. See also Fig. S3 in the supplemental material.
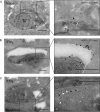
FIG 4
LC3 and LAMP-1 localization in cells infected with T. gondii (Tg). Cryo-immuno-EM localization of GFP-LC3 and LAMP-1 in naive (A) or IFN-γ (5 ng/ml)-activated (B, C) HeLa cells infected with type 3 (VEG) parasites at 6 h postinfection. (A, B) GFP-LC3 was localized with a rabbit polyclonal antibody to GFP, followed by goat anti-rabbit IgG conjugated to 18-nm gold. (C) LAMP-1 was localized with rabbit MAb D2D11, followed by donkey anti-rabbit IgG conjugated to 12-nm gold (small white arrowheads), GFP-LC3 was localized with a goat polyclonal antibody to GFP, followed by donkey anti-goat IgG conjugated to 18-nm gold (large white arrowheads). Arrowheads indicate parasite membranes, arrows indicate the PVM, and asterisks indicate additional membranes. Scale bars, 500 nm. See also Fig. S4 in the supplemental material.
FIG 5
Impaired replication of T. gondii in vacuoles targeted by autophagy adaptors. Quantification of parasite replication in vacuoles positive for ubiquitin (A) or p62 (C). The number of parasites per vacuole was assessed by immunostaining for parasite surface or host cell markers, followed by epifluorescence microscopy for type 3 (VEG) parasites in IFN-γ-activated HeLa cells at 24 h postinfection. Each value is the mean ± SEM of three experiments (***, P < 0.001; two-way ANOVA). (B, D) Representative immunofluorescence images of vacuoles positive or negative for ubiquitin or p62. (B) Ubiquitin was localized with mouse MAb FK2, followed by goat-anti mouse IgG conjugated to Alexa Fluor 594 (red). Parasites were localized with a rabbit polyclonal antibody to tachyzoites, followed by goat anti-rabbit IgG conjugated to Alexa Fluor 488 (green). (D) p62 was localized with a guinea pig polyclonal antibody, followed by anti-guinea pig IgG conjugated to Alexa Fluor 594 (red). T. gondii (αTg) was localized with MAb DG52 to SAG1, followed by goat anti-mouse IgG conjugated to Alexa Fluor 488 (green). Nuclei were stained with DAPI. Scale bars, 5 µm. (E) Quantification of recruitment of ubiquitin to type 1 (GT-1), 2 (PTG), and 3 (VEG) parasites at 6 or 24 h postinfection in IFN-γ-activated HeLa cells. Each value is the mean ± SEM of three experiments (**, P < 0.01; *, P < 0.05; one-way ANOVA). Strains were compared to each other at the same time point. (F) Quantification of parasite replication of type 1 (GT-1), 2 (PTG), and 3 (VEG) parasites in vacuoles positive or negative for ubiquitin at 24 h postinfection in IFN-γ-activated HeLa cells. Replication was quantified as described above. The total numbers of positive vacuoles counted for replication were as follows: type 1, 90; type 2, 450; type 3, 450 (**, P < 0.01; *, P < 0.05; two-way ANOVA). See also Fig. S5 in the supplemental material.
FIG 6
Replication of T. gondii in autophagy-deficient HeLa cells. (A) Replication of type 3 (VEG) T. gondii in vacuoles positive for ubiquitin in IFN-γ-activated wild-type (WT), ATG16L1 KO (clone E6), or ATG16L1-complemented (WT/Comp or E6/comp) host cell strains at 24 h postinfection. Each value is the mean ± SEM of three experiments (***, P < 0.001; two-way ANOVA). (B) Representative fluorescence images of T. gondii vacuoles in ATG16L1 KO or complemented strains. Ubiquitin was localized with mouse MAb FK2, followed by goat anti-mouse IgG conjugated to Alexa Fluor 594 (red). Parasites were localized with a rabbit polyclonal antibody to tachyzoites (αTg), followed by goat anti-rabbit IgG conjugated to Alexa Fluor 488 (green). Scale bars, 5 µm. (C) Replication of type 3 (VEG) T. gondii positive for ubiquitin in IFN-γ-activated ATG7 KO HeLa cells at 24 h postinfection. Each value is the mean ± SEM of three experiments (***, P < 0.001; two-way ANOVA). (D) Representative images of T. gondii vacuoles in wild-type (WT) HeLa cells or ATG7 KO clones (1A6 or 1A11). Ubiquitin was localized with mouse MAb FK2, followed by goat anti-mouse IgG conjugated to Alexa Fluor 594 (red). Parasites were localized with a rabbit polyclonal antibody to tachyzoites (αTg), followed by goat anti-rabbit IgG conjugated to Alexa Fluor 488 (green). Scale bars, 5 µm. (E) Quantification of recruitment of ubiquitin to type 3 (VEG) parasites in IFN-γ-activated (5 ng/ml) U2OS cells treated with tetracycline-inducible shRNA in the presence or absence of tetracycline at 6 h postinfection. Each value is the mean ± SEM of three experiments (n.s., not significant; two-way ANOVA). (F) Expression of Beclin 1 and ATG14 detected by Western blotting of lysates from parental U2OS cells or ± tetracycline-induced shRNA knockdown of Beclin 1 and ATG14. Beclin 1 and ATG14 were detected with a rabbit MAb (Beclin 1) or a rabbit polyclonal antibody (ATG14), followed by LiCor IRDye 800CW goat anti-rabbit IgG (green). Actin was used as a loading control and detected with mouse MAb C4, followed by LiCor IRDye 680CW goat anti-mouse IgG (red). Arrowheads indicate Beclin 1 or ATG14 protein. The values to the left are molecular sizes in kilodaltons. (G) Quantification of recruitment of ubiquitin to type 3 (VEG) parasites in naive or IFN-γ-activated HeLa cells treated with 25 µM Tat-Beclin 1, Tat-scrambled peptide, or Opti-MEM at 6 h postinfection. Each value is the mean ± SD of two experiments with three internal replicates and a total of six coverslips. n.s., not significant; Kruskal-Wallis test. (H) Representative fluorescence images of HeLa cells expressing LC3-GFP treated with 25 µM Tat-Beclin 1 or Tat-scrambled peptide for 3 h. GFP-LC3 was localized with mouse MAb 3E6 against GFP, followed by goat anti-mouse IgG conjugated to Alexa Fluor 488. Scale bar, 20 nm. See also Fig. S6 in the supplemental material.
Similar articles
- ISG15 Connects Autophagy and IFN-γ-Dependent Control of Toxoplasma gondii Infection in Human Cells.
Bhushan J, Radke JB, Perng YC, Mcallaster M, Lenschow DJ, Virgin HW, Sibley LD. Bhushan J, et al. mBio. 2020 Oct 6;11(5):e00852-20. doi: 10.1128/mBio.00852-20. mBio. 2020. PMID: 33024031 Free PMC article. - Role of mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii.
Ohshima J, Lee Y, Sasai M, Saitoh T, Su Ma J, Kamiyama N, Matsuura Y, Pann-Ghill S, Hayashi M, Ebisu S, Takeda K, Akira S, Yamamoto M. Ohshima J, et al. J Immunol. 2014 Apr 1;192(7):3328-35. doi: 10.4049/jimmunol.1302822. Epub 2014 Feb 21. J Immunol. 2014. PMID: 24563254 - K63-Linked Ubiquitination Targets Toxoplasma gondii for Endo-lysosomal Destruction in IFNγ-Stimulated Human Cells.
Clough B, Wright JD, Pereira PM, Hirst EM, Johnston AC, Henriques R, Frickel EM. Clough B, et al. PLoS Pathog. 2016 Nov 22;12(11):e1006027. doi: 10.1371/journal.ppat.1006027. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27875583 Free PMC article. - Quo vadis? Interferon-inducible GTPases go to their target membranes via the LC3-conjugation system of autophagy.
Choi J, Biering SB, Hwang S. Choi J, et al. Small GTPases. 2017 Oct 2;8(4):199-207. doi: 10.1080/21541248.2016.1213090. Epub 2016 Jul 18. Small GTPases. 2017. PMID: 27428166 Free PMC article. Review. - The Host Autophagy During Toxoplasma Infection.
Wu M, Cudjoe O, Shen J, Chen Y, Du J. Wu M, et al. Front Microbiol. 2020 Oct 22;11:589604. doi: 10.3389/fmicb.2020.589604. eCollection 2020. Front Microbiol. 2020. PMID: 33193253 Free PMC article. Review.
Cited by
- Locally generated C3 regulates the clearance of Toxoplasma gondii by IFN-γ-primed macrophage through regulation of xenophagy.
Liu B, Yan Y, Wang X, Chen N, Wu J. Liu B, et al. Front Microbiol. 2022 Aug 4;13:944006. doi: 10.3389/fmicb.2022.944006. eCollection 2022. Front Microbiol. 2022. PMID: 35992649 Free PMC article. - The Protective Role of TLR2 Mediates Impaired Autophagic Flux by Activating the mTOR Pathway During Neospora caninum Infection in Mice.
Wang J, Wang X, Gong P, Ren F, Li X, Zhang N, Zhang X, Zhang X, Li J. Wang J, et al. Front Cell Infect Microbiol. 2021 Nov 26;11:788340. doi: 10.3389/fcimb.2021.788340. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34900761 Free PMC article. - Toxoplasma gondii infection and its implications within the central nervous system.
Matta SK, Rinkenberger N, Dunay IR, Sibley LD. Matta SK, et al. Nat Rev Microbiol. 2021 Jul;19(7):467-480. doi: 10.1038/s41579-021-00518-7. Epub 2021 Feb 24. Nat Rev Microbiol. 2021. PMID: 33627834 Review. - Exposing Toxoplasma gondii hiding inside the vacuole: a role for GBPs, autophagy and host cell death.
Saeij JP, Frickel EM. Saeij JP, et al. Curr Opin Microbiol. 2017 Dec;40:72-80. doi: 10.1016/j.mib.2017.10.021. Epub 2017 Nov 12. Curr Opin Microbiol. 2017. PMID: 29141239 Free PMC article. Review. - Overview of Apoptosis, Autophagy, and Inflammatory Processes in Toxoplasma gondii Infected Cells.
Ahmadpour E, Babaie F, Kazemi T, Mehrani Moghaddam S, Moghimi A, Hosseinzadeh R, Nissapatorn V, Pagheh AS. Ahmadpour E, et al. Pathogens. 2023 Feb 4;12(2):253. doi: 10.3390/pathogens12020253. Pathogens. 2023. PMID: 36839525 Free PMC article. Review.
References
- Dubey JP. 2010. Toxoplasmosis of animals and humans. CRC Press, Boca Raton, FL.
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI109725/AI/NIAID NIH HHS/United States
- R01 AI036629/AI/NIAID NIH HHS/United States
- AI118426/AI/NIAID NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- T32 AI007163/AI/NIAID NIH HHS/United States
- R01 AI118426/AI/NIAID NIH HHS/United States
- AI036629/AI/NIAID NIH HHS/United States
- R01 DK097485/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous