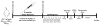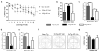Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer's mice - PubMed (original) (raw)
Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer's mice
Angélica Maria Sabogal-Guáqueta et al. Neuropharmacology. 2016 Mar.
Abstract
Alzheimer's disease (AD) is an age-related progressive neurodegenerative disorder. Several types of treatments have been tested to block or delay the onset of the disease, but none have been completely successful. Diet, lifestyle and natural products are currently the main scientific focuses. Here, we evaluate the effects of oral administration of the monoterpene linalool (25 mg/kg), every 48 h for 3 months, on aged (21-24 months old) mice with a triple transgenic model of AD (3xTg-AD) mice. Linalool-treated 3xTg-AD mice showed improved learning and spatial memory and greater risk assessment behavior during the elevated plus maze. Hippocampi and amygdalae from linalool-treated 3xTg-AD mice exhibited a significant reduction in extracellular β-amyloidosis, tauopathy, astrogliosis and microgliosis as well as a significant reduction in the levels of the pro-inflammatory markers p38 MAPK, NOS2, COX2 and IL-1β. Together, our findings suggest that linalool reverses the histopathological hallmarks of AD and restores cognitive and emotional functions via an anti-inflammatory effect. Thus, linalool may be an AD prevention candidate for preclinical studies.
Keywords: Alzheimer's disease; Behavior function; Inflammation; Linalool.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflicts of interest.
Figures
Figure 1. Experimental design
Linalool (25 mg/kg) or saline were administered orally by gavage to 18- to 21-month-old Non-Tg and 3xTg-AD mice for 3 months, every 48 hours. Learning and memory were evaluated in the Morris water maze test (five days, ten trials) at 21–24 months of age. Afterward, the elevated plus maze was performed over two days. Then, the mice were sacrificed for histological and biochemical analyses.
Figure 2. Linalool treatment prevented spatial memory impairment in 3xTg- AD mice
a) Mean latency to reach the hidden platform during the spatial learning task. b) Latency, c) number of crossings, d) time spent reaching the platform and e) distance to reach the platform in the retention test. f) Representative images of the route of travel during the retention test. g) No differences were detected between the experimental groups in the visual, motor or motivational skills of the animals during the visible test. The data are expressed as the means ± SEM. n=8–16. *Difference between the 3xTg-AD vehicle-treated group and the other groups *p<0.05.
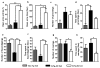
Figure 3. Anxiolytic effect of linalool oral administration on 3xTg-AD mice
ab) Relative frequency of open arm entries and the time spent in the open arms. c–d) Number of grooming behaviors and the time spent grooming. e–f) Number of head-dipping behaviors and the time spent head-dipping. g–h) Number of rearing actions and the time spent rearing at the end of the EPM test performed two weeks after the final dose of vehicle or linalool. The data are expressed as the means ± SEM. n=4–5. *p<0.05.
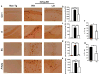
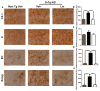
Figure 4. 3xTg-AD linalool-treated mice show reduced β-amyloidosis
a) Representative images of βA (anti-βA 6E10) immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of vehicle- and linalool-treated 3xTg-AD and vehicle-treated Non-Tg mice at 21–24 months of age. Magnification: 10x; scale bar: 50 μm. b) The values in the bar graph are expressed in densitometric relative units (RU) of βA immunoreactivity in the CA1 area, c) he subiculum, d) the EC and e) the amygdala. f) The relative βA 1–40 and g) βA 1–42 fragment levels were analyzed in hippocampal lysates by ELISA. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4–5. *p<0.05; **p<0.01; ***p<0.001.
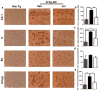
Figure 5. Linalool treatment attenuated tau hyperphosphorylation in old 3xTg-AD mice
a) Representative images of AT8 (anti-tau pSer202/Thr205) immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non-Tg and 3xTg-AD mice treated with vehicle or linalool. Magnification: 10x; scale bar: 50 μm. b) The values in the bar graph are expressed in densitometric relative units (RU) of AT8 immunoreactivity in the CA1 area, c) the subiculum, d) the EC and e) the amygdala. f) Representative bands and densitometric intensities of PHF-1 in hippocampal lysates. Tubulin was used as a loading control. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4. *p<0.05; **p<0.01; ***p<0.001.
Figure 6. Linalool decreased astrogliosis in old 3xTg-AD mice
a) Representative images of GFAP immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non-Tg and 3xTg-AD mice treated with vehicle or Linalool. Magnification: 40x. b) The values in the bar graphs are expressed in densitometric relative units (RU) of GFAP immunoreactivity in the CA1 area, c) the subiculum, d) the EC and e) the amygdala. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=3–4. **p<0.01; ***p<0.001.
Figure 7. Linalool ameliorated microgliosis in 3xTg-AD mice
a) Representative images of GFAP immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non-Tg and 3xTg-AD mice treated with vehicle or linalool. Magnification: 40x. b) The values in the bar graphs are expressed in densitometric relative units (RU) of Iba1 immunoreactivity in the CA1 area, c) the subiculum, d) the EC and e) the amygdala. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=3–4. *p<0.05 **p 0.01; ***p<0.001.
Figure 8. Linalool reduced the proinflammatory response in 3xTg-AD mice
Morphological characterization showing nuclei stained with Hoechst (blue), β-amyloid stained with Alexa Fluor 594 phalloidin dye (red) and microglia visualized with Iba1 (green). Magnification, 10x and 60x. a) Representative images of CA1, subiculum, EC, and the amygdala. b) Quantification of IL-1β in hippocampal lysates. Representative bands and densitometric intensities of c) COX-2, d) NOS2 and e) p38 MAPK in hippocampal lysates. Veh: vehicle (Saline solution); Lin: Linalool; CA1 and S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4–5. *p<0.05; **p<0.01‚ ***p<0.001.
Similar articles
- The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice.
Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. Sabogal-Guáqueta AM, et al. Neuropharmacology. 2015 Jun;93:134-45. doi: 10.1016/j.neuropharm.2015.01.027. Epub 2015 Feb 7. Neuropharmacology. 2015. PMID: 25666032 Free PMC article. - SLOH, a carbazole-based fluorophore, mitigates neuropathology and behavioral impairment in the triple-transgenic mouse model of Alzheimer's disease.
Wu X, Kosaraju J, Zhou W, Tam KY. Wu X, et al. Neuropharmacology. 2018 Mar 15;131:351-363. doi: 10.1016/j.neuropharm.2018.01.003. Epub 2018 Jan 5. Neuropharmacology. 2018. PMID: 29309769 - Mechanisms of Hydrogen Sulfide against the Progression of Severe Alzheimer's Disease in Transgenic Mice at Different Ages.
Vandini E, Ottani A, Zaffe D, Calevro A, Canalini F, Cavallini GM, Rossi R, Guarini S, Giuliani D. Vandini E, et al. Pharmacology. 2019;103(1-2):50-60. doi: 10.1159/000494113. Epub 2018 Nov 16. Pharmacology. 2019. PMID: 30448835 - Preventive Effect of Quercetin in a Triple Transgenic Alzheimer's Disease Mice Model.
Paula PC, Angelica Maria SG, Luis CH, Gloria Patricia CG. Paula PC, et al. Molecules. 2019 Jun 20;24(12):2287. doi: 10.3390/molecules24122287. Molecules. 2019. PMID: 31226738 Free PMC article. - Neuroprotective Effect of SLM, a Novel Carbazole-Based Fluorophore, on SH-SY5Y Cell Model and 3xTg-AD Mouse Model of Alzheimer's Disease.
Wu X, Kosaraju J, Zhou W, Tam KY. Wu X, et al. ACS Chem Neurosci. 2017 Mar 15;8(3):676-685. doi: 10.1021/acschemneuro.6b00388. Epub 2016 Dec 29. ACS Chem Neurosci. 2017. PMID: 28032988
Cited by
- Mechanistic Insights into the Neuroprotective Potential of Sacred Ficus Trees.
Shim KH, Sharma N, An SSA. Shim KH, et al. Nutrients. 2022 Nov 9;14(22):4731. doi: 10.3390/nu14224731. Nutrients. 2022. PMID: 36432418 Free PMC article. Review. - Maternal linalool treatment protects against radiofrequency wave-induced deteriorations in adolescent rats: A behavioral and electrophysiological study.
Azimzadeh M, Noorbakhshnia M. Azimzadeh M, et al. Sci Rep. 2024 Jul 27;14(1):17257. doi: 10.1038/s41598-024-68103-5. Sci Rep. 2024. PMID: 39060318 Free PMC article. - Lavandula angustifolia Essential Oil and Linalool Counteract Social Aversion Induced by Social Defeat.
Caputo L, Reguilon MD, Mińarro J, De Feo V, Rodriguez-Arias M. Caputo L, et al. Molecules. 2018 Oct 19;23(10):2694. doi: 10.3390/molecules23102694. Molecules. 2018. PMID: 30347669 Free PMC article. - Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics.
Sharifi-Rad M, Lankatillake C, Dias DA, Docea AO, Mahomoodally MF, Lobine D, Chazot PL, Kurt B, Tumer TB, Moreira AC, Sharopov F, Martorell M, Martins N, Cho WC, Calina D, Sharifi-Rad J. Sharifi-Rad M, et al. J Clin Med. 2020 Apr 8;9(4):1061. doi: 10.3390/jcm9041061. J Clin Med. 2020. PMID: 32276438 Free PMC article. Review. - Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review.
Dos Santos ÉRQ, Maia JGS, Fontes-Júnior EA, do Socorro Ferraz Maia C. Dos Santos ÉRQ, et al. Curr Neuropharmacol. 2022;20(6):1073-1092. doi: 10.2174/1570159X19666210920094504. Curr Neuropharmacol. 2022. PMID: 34544345 Free PMC article. Review.
References
- Anjos PJ, Lima AO, Cunha PS, De Sousa DP, Onofre AS, Ribeiro TP, Medeiros IA, Antoniolli AR, Quintans-Junior LJ, Santosa MR. Cardiovascular effects induced by linalool in normotensive and hypertensive rats. Z Naturforsch C. 2013;68:181–190. - PubMed
- Association, A. s. Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia. 2014;10 - PubMed
- Azizi Z, Ebrahimi S, Saadatfar E, Kamalinejad M, Majlessi N. Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol. 2012;23:241–249. - PubMed
- Bassil N, Grossberg GT. Novel regimens and delivery systems in the pharmacological treatment of Alzheimer’s disease. CNS Drugs. 2009;23:293–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials