Sensitivity of Human Intrahepatic Cholangiocarcinoma Subtypes to Chemotherapeutics and Molecular Targeted Agents: A Study on Primary Cell Cultures - PubMed (original) (raw)
. 2015 Nov 16;10(11):e0142124.
doi: 10.1371/journal.pone.0142124. eCollection 2015.
Vincenzo Cardinale 1, Maria Consiglia Bragazzi 1, Felice Giuliante 2, Agostino Maria De Rose 2, Gian Luca Grazi 3, Chiara Napoletano 4, Rossella Semeraro 1, Anna Maria Lustri 1, Daniele Costantini 1, Lorenzo Nevi 1, Sabina Di Matteo 1, Anastasia Renzi 5, Guido Carpino [ 6](#full-view-affiliation-6 "Health Science, University of Rome "Foro Italico", Rome, Italy."), Eugenio Gaudio 5, Domenico Alvaro 1
Affiliations
- PMID: 26571380
- PMCID: PMC4646673
- DOI: 10.1371/journal.pone.0142124
Sensitivity of Human Intrahepatic Cholangiocarcinoma Subtypes to Chemotherapeutics and Molecular Targeted Agents: A Study on Primary Cell Cultures
Alice Fraveto et al. PLoS One. 2015.
Abstract
We investigated the sensitivity of intrahepatic cholangiocarcinoma (IHCCA) subtypes to chemotherapeutics and molecular targeted agents. Primary cultures of mucin- and mixed-IHCCA were prepared from surgical specimens (N. 18 IHCCA patients) and evaluated for cell proliferation (MTS assay) and apoptosis (Caspase 3) after incubation (72 hours) with increasing concentrations of different drugs. In vivo, subcutaneous human tumor xenografts were evaluated. Primary cultures of mucin- and mixed-IHCCA were characterized by a different pattern of expression of cancer stem cell markers, and by a different drug sensitivity. Gemcitabine and the Gemcitabine-Cisplatin combination were more active in inhibiting cell proliferation in mixed-IHCCA while Cisplatin or Abraxane were more effective against mucin-IHCCA, where Abraxane also enhances apoptosis. 5-Fluoracil showed a slight inhibitory effect on cell proliferation that was more significant in mixed- than mucin-IHCCA primary cultures and, induced apoptosis only in mucin-IHCCA. Among Hg inhibitors, LY2940680 and Vismodegib showed slight effects on proliferation of both IHCCA subtypes. The tyrosine kinase inhibitors, Imatinib Mesylate and Sorafenib showed significant inhibitory effects on proliferation of both mucin- and mixed-IHCCA. The MEK 1/2 inhibitor, Selumetinib, inhibited proliferation of only mucin-IHCCA while the aminopeptidase-N inhibitor, Bestatin was more active against mixed-IHCCA. The c-erbB2 blocking antibody was more active against mixed-IHCCA while, the Wnt inhibitor, LGK974, similarly inhibited proliferation of mucin- and mixed-IHCCA. Either mucin- or mixed-IHCCA showed high sensitivity to nanomolar concentrations of the dual PI3-kinase/mTOR inhibitor, NVP-BEZ235. In vivo, in subcutaneous xenografts, either NVP-BEZ235 or Abraxane, blocked tumor growth. In conclusion, mucin- and mixed-IHCCA are characterized by a different drug sensitivity. Cisplatin, Abraxane and the MEK 1/2 inhibitor, Selumetinib were more active against mucin-IHCCA while, Gemcitabine, Gemcitabine-Cisplatin combination, the c-erbB2 blocking antibody and bestatin worked better against mixed-IHCCA. Remarkably, we identified a dual PI3-kinase/mTOR inhibitor that both in vitro and in vivo, exerts dramatic antiproliferative effects against both mucin- and mixed-IHCCA.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Fig 1. Characterization of mixed- and mucin-IHCCA primary cultures.
(A) Immunohistochemical and immunofluorescence analyses of mixed- and mucin-IHCCA primary cultures for Vimentin, α-SMA, E-Cadherin and the “epithelial” cancer stem cell markers CD133+, EpCAM+, LGR5+ and for IL6. A diffuse positivity for mesenchymal markers (Vimentin, α-SMA) and IL6 was observed while E-Cadherin was virtually negative and less than 5% cells where positive for the “epithelial” cancer stem cell markers. Representative experiment of N = 12 independent staining performed in separate primary cultures. (B) Flow cytometry analyses of primary cultures of mixed- and mucin-IHCCA (20–30 passages), labeled with anti-CD13, anti-CD90, anti-EpCAM, anti-CD133, and anti-LGR5 antibodies. Bar graphs and representative plots. Cells positive for CD13 and CD90 largely predominated with respect to CD133, EpCAM and LGR5. CD13+ cells predominated in mixed-IHCCA with respect to mucin-IHCCA, while the opposite was found for CD90+ cells. Mean ± SD of N = 18 independent experiments. * = p< 0.05 vs mucin-IHCCA; & = p< 0.01 vs mixed-IHCCA.
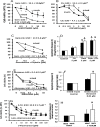
Fig 2. Effects of chemotherapeutics (Gemcitabine, Cisplatin, Abraxane) on proliferation and apoptosis of mucin- and mixed-CCA primary cultures.
Cell proliferation was evaluated by MTS assay and results were expressed as percentage with respect to controls considered equal to 100. Apoptosis was evaluated by measuring caspase-3 activity and expressed as ratio between drug-treated and control cells. MTS and caspase-3 were measured 72 hours after incubation with the tested drug. Gemcitabine was much more active in inhibiting cell proliferation (A) (MTS assay) in mixed-IHCCA than mucin-IHCCA primary cultures. Cisplatin (B), in contrast, was more active against mucin-IHCCA. With respect to the two drugs alone, the Gemcitabile-Cisplatin combination induced a higher inhibition of cell proliferation (C) in mucin-IHCCA but not in mixed-IHCCA. Gemcitabine but not Cisplatin significantly enhanced Caspase-3 activity (D) without differences between mucin- and mixed-IHCCA and, the combination Gem+Cis does not further enhances the apoptotic effects of Gemcitabine alone. Abraxane showed a significant inhibitory effect on cell proliferation (E) in both mixed- and mucin-IHCCA primary cultures, although the effect on mucin-IHCCA was predominant (p< 0.05). Abraxane induced a significant increase of apoptosis only in mucin-IHCCA (F). 5-FU slightly inhibited cell proliferation (G) with a more significant effect on mixed- than mucin-IHCCA (p<0.05). 5-FU induced a significant increase of apoptosis only in mucin-IHCCA (H). *p< 0.05 mixed vs mucin. & = p< 0.05 vs controls. Mean ± SD of N = 5–7 independent experiments.
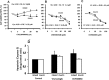
Fig 3. Effect of Sonic Hedgehog (Hg) pathway inhibitors on proliferation and apoptosis of mucin and mixed CCA primary cultures.
Cyclopamine (A) displayed no effect on cell proliferation in primary cultures of both mucin- and mixed-IHCCAs. In contrast, Vismodegib (B) and LY2940680 (C), showed a slight inhibitory effect on cell proliferation in both CCAs subtypes. Apoptosis was unaffected by Vismodegib or LY2940680. N experiments = 5–7.
Fig 4. Effect of tyrosine kinase inhibitors on proliferation and apoptosis of mucin and mixed CCA primary cultures.
Genistein showed no effect on cell proliferation (A). Imatinib mesylate (B) and Sorafenib (C) inhibited cell proliferation in mucin-IHCCA and mixed-IHCCA but only the former enhance apoptosis (D) and only in mixed-IHCCA. *p< 0.05 vs controls. N experiments = 5–7.
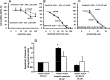
Fig 5. Effect of Epidermal growth factor receptor (EGFR) antagonists and PI3-kinase/AKT inhibitors on proliferation and apoptosis of mucin and mixed CCA primary cultures.
The EGFR antagonist, cetuximab (A), had no effect on cell proliferation neither on mucin- nor in mixed-IHCCA. In contrast, the c-erbB2 blocking antibody (B) inhibited proliferation in both mucin- and mixed-IHCCA with a predominant effect on the latter. Cetuximab and the c-erbB2 blocking antibody showed no effect on cell apoptosis (C). *p< 0.05 mucin vs mixed, N experiments = 5–7. The PI3-kinase/AKT inhibitors, NVP-BEZ235 (D) and MK2206 (E) showed strong inhibitory effect on cell proliferation in both mucin- and mixed-IHCCA primary cultures, but only MK2206 enhanced apoptosis (F) without differences between mucin-IHCCA and mixed-IHCCA. *p< 0.05 mucin vs mixed, & = p< 0.05 vs controls. N experiments = 5–7.
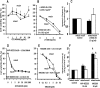
Fig 6. Effect of MEK 1/2 inhibitor, Aminopeptidase-N inhibitor and Wnt inhibitor on proliferation and apoptosis of mucin and mixed CCA primary cultures.
The MEK 1/2 inhibitor, selumetinib (AZD6244) (A), inhibits cell proliferation only at high concentrations and with a predominant effect on mucin-IHCCA. Apoptosis (B) was enhanced only in mucin-IHCCA. *p< 0.05 mucin vs mixed, & = p< 0.05 vs controls. N experiments = 5–7. The aminopeptidase-N inhibitor, bestatin (C), showed an inhibitory effect on cell proliferation that predominated in mixed-IHCCA. *p< 0.05 mucin vs mixed, N experiments = 5–7. The Wnt inhibitor, LGK974, (D) starting from 10 μM concentration inhibited proliferation of both mucin- and mixed-IHCCA, N experiments = 3–5.
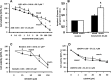
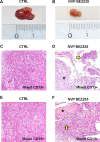
Fig 7. Effect of NVP-BEZ235 on subcutaneous human tumor xenografts.
In control mice (CTRL), the tumor volume of subcutaneous xenografts increased during four weeks of observation (A) while in mice treated for 2 weeks with NVP-BEZ235 (B) it remains almost stable. At the histo-pathological evaluation (Hematoxylin-Eosin), in control mice (CTRL), subcutaneous masses arisen after the implantation of CD13+ spheroids from mixed- (C) or mucin-IHCCA (E) cultures, were composed of densely packed tumor cells. Original Magnification(OM): 10x. C, E). In mice treated with NVP-BEZ235, subcutaneous xenografts arisen from CD13+ spheroids, immunosorted from mixed-IHCCA cultures (D) were characterized by extensive necrosis (asterisk) and few tumoral cells (arrow); necrosis was less extended in masses arisen after the implantation of CD13+spheroids immunosorted from mucin-IHCCAs (F, asterisk = necrosis, arrow = tumoral cells). OM: 10x (D, F).
Similar articles
- Combination of anti-L1 cell adhesion molecule antibody and gemcitabine or cisplatin improves the therapeutic response of intrahepatic cholangiocarcinoma.
Cho S, Lee TS, Song IH, Kim AR, Lee YJ, Kim H, Hwang H, Jeong MS, Kang SG, Hong HJ. Cho S, et al. PLoS One. 2017 Feb 6;12(2):e0170078. doi: 10.1371/journal.pone.0170078. eCollection 2017. PLoS One. 2017. PMID: 28166242 Free PMC article. - Metformin potentiates the anticancer activities of gemcitabine and cisplatin against cholangiocarcinoma cells in vitro and in vivo.
Zhu HQ, Ma JB, Song X, Gao HJ, Ma CQ, Chang H, Li HG, Liu FF, Lu J, Zhou X. Zhu HQ, et al. Oncol Rep. 2016 Dec;36(6):3488-3496. doi: 10.3892/or.2016.5187. Epub 2016 Oct 20. Oncol Rep. 2016. PMID: 27779693 - Gefitinib and gemcitabine coordinately inhibited the proliferation of cholangiocarcinoma cells.
Nakajima Y, Takagi H, Kakizaki S, Horiguchi N, Sato K, Sunaga N, Mori M. Nakajima Y, et al. Anticancer Res. 2012 Dec;32(12):5251-62. Anticancer Res. 2012. PMID: 23225424 - Systemic therapy of cholangiocarcinoma: From chemotherapy to targeted therapies.
Schweitzer N, Vogel A. Schweitzer N, et al. Best Pract Res Clin Gastroenterol. 2015 Apr;29(2):345-53. doi: 10.1016/j.bpg.2015.01.002. Epub 2015 Feb 19. Best Pract Res Clin Gastroenterol. 2015. PMID: 25966433 Review. - Recent Advances in Systemic Therapy for Advanced Intrahepatic Cholangiocarcinoma.
Yoo C, Hyung J, Chan SL. Yoo C, et al. Liver Cancer. 2023 Jun 8;13(2):119-135. doi: 10.1159/000531458. eCollection 2024 Apr. Liver Cancer. 2023. PMID: 38638168 Free PMC article. Review.
Cited by
- Thymoquinone inhibited vasculogenic capacity and promoted mesenchymal-epithelial transition of human breast cancer stem cells.
Haiaty S, Rashidi MR, Akbarzadeh M, Bazmani A, Mostafazadeh M, Nikanfar S, Zibaei Z, Rahbarghazi R, Nouri M. Haiaty S, et al. BMC Complement Med Ther. 2021 Mar 4;21(1):83. doi: 10.1186/s12906-021-03246-w. BMC Complement Med Ther. 2021. PMID: 33663486 Free PMC article. - Protective autophagy or autophagic death: effects of BEZ235 on chronic myelogenous leukemia.
Xin P, Xu W, Zhu X, Li C, Zheng Y, Zheng T, Cheng W, Peng Q. Xin P, et al. Cancer Manag Res. 2019 Aug 22;11:7933-7951. doi: 10.2147/CMAR.S204472. eCollection 2019. Cancer Manag Res. 2019. PMID: 31686909 Free PMC article. - Impact of Aberrant β-Catenin Pathway on Cholangiocarcinoma Heterogeneity.
Lozano E, Sanchon-Sanchez P, Morente-Carrasco A, Chinchilla-Tábora LM, Mauriz JL, Fernández-Palanca P, Marin JJG, Macias RIR. Lozano E, et al. Cells. 2023 Apr 12;12(8):1141. doi: 10.3390/cells12081141. Cells. 2023. PMID: 37190050 Free PMC article. Review. - DCLK1, a Putative Stem Cell Marker in Human Cholangiocarcinoma.
Nevi L, Di Matteo S, Carpino G, Zizzari IG, Samira S, Ambrosino V, Costantini D, Overi D, Giancotti A, Monti M, Bosco D, De Peppo V, Oddi A, De Rose AM, Melandro F, Bragazzi MC, Faccioli J, Massironi S, Grazi GL, Panici PB, Berloco PB, Giuliante F, Cardinale V, Invernizzi P, Caretti G, Gaudio E, Alvaro D. Nevi L, et al. Hepatology. 2021 Jan;73(1):144-159. doi: 10.1002/hep.31571. Hepatology. 2021. PMID: 32978808 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
Funded by University “Sapienza” of Rome (DA, EG) and Consorzio Interuniversitario Trapianti d'Organo (DA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous