UV Damage-Induced Phosphorylation of HBO1 Triggers CRL4DDB2-Mediated Degradation To Regulate Cell Proliferation - PubMed (original) (raw)
. 2015 Nov 16;36(3):394-406.
doi: 10.1128/MCB.00809-15. Print 2016 Feb 1.
Hiroyuki Niida 2, Tatsuya Ohhata 3, Kyoko Kitagawa 3, Satoshi Sakai 3, Chiharu Uchida 4, Bunsyo Shiotani 5, Masaki Matsumoto 6, Keiichi I Nakayama 6, Hiroyuki Ogura 7, Norihiko Shiiya 7, Masatoshi Kitagawa 3
Affiliations
- PMID: 26572825
- PMCID: PMC4719422
- DOI: 10.1128/MCB.00809-15
UV Damage-Induced Phosphorylation of HBO1 Triggers CRL4DDB2-Mediated Degradation To Regulate Cell Proliferation
Ryoichi Matsunuma et al. Mol Cell Biol. 2015.
Erratum in
- Correction for Matsunuma et al., "UV Damage-Induced Phosphorylation of HBO1 Triggers CRL4DDB2-Mediated Degradation To Regulate Cell Proliferation".
Matsunuma R, Niida H, Ohhata T, Kitagawa K, Sakai S, Uchida C, Shiotani B, Matsumoto M, Nakayama KI, Ogura H, Shiiya N, Kitagawa M. Matsunuma R, et al. Mol Cell Biol. 2018 Mar 15;38(7):e00572-17. doi: 10.1128/MCB.00572-17. Print 2018 Apr 1. Mol Cell Biol. 2018. PMID: 29545385 Free PMC article. No abstract available.
Abstract
Histone acetyltransferase binding to ORC-1 (HBO1) is a critically important histone acetyltransferase for forming the prereplicative complex (pre-RC) at the replication origin. Pre-RC formation is completed by loading of the MCM2-7 heterohexameric complex, which functions as a helicase in DNA replication. HBO1 recruited to the replication origin by CDT1 acetylates histone H4 to relax the chromatin conformation and facilitates loading of the MCM complex onto replication origins. However, the acetylation status and mechanism of regulation of histone H3 at replication origins remain elusive. HBO1 positively regulates cell proliferation under normal cell growth conditions. Whether HBO1 regulates proliferation in response to DNA damage is poorly understood. In this study, we demonstrated that HBO1 was degraded after DNA damage to suppress cell proliferation. Ser50 and Ser53 of HBO1 were phosphorylated in an ATM/ATR DNA damage sensor-dependent manner after UV treatment. ATM/ATR-dependently phosphorylated HBO1 preferentially interacted with DDB2 and was ubiquitylated by CRL4(DDB2). Replacement of endogenous HBO1 in Ser50/53Ala mutants maintained acetylation of histone H3K14 and impaired cell cycle regulation in response to UV irradiation. Our findings demonstrate that HBO1 is one of the targets in the DNA damage checkpoint. These results show that ubiquitin-dependent control of the HBO1 protein contributes to cell survival during UV irradiation.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
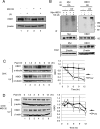
FIG 1
HBO1 is degraded by the ubiquitin-proteasome system in response to UV damage. (A) Endogenous HBO1 was degraded by UV light and protected by MG132. HEK293 cells were treated with or without MG132 (10 μM) for 4 h, followed by irradiation with 40 J/m2 UV light, and were then cultured for 3 h. Cell lysates were analyzed by Western blotting with the indicated antibodies. (B) UV-induced ubiquitylation of HBO1 was detected under denaturing conditions. HEK293 cells were transfected with Myc-HBO1 WT and HA-Ub (left) or only with HA-Ub (right) and then treated with MG132 for 4 h before UV irradiation at 40 J/m2 and were then cultured for 2 h. Cell lysates were immunoprecipitated with anti-Myc antibody (left) or anti-HBO1 antibody (right) under denaturing conditions, and the immunoprecipitates were examined by Western blotting (IB) using the indicated antibodies. The asterisk indicates a nonspecific band. (C) Endogenous HBO1 was degraded after UV irradiation. HEK293 cells were irradiated with 100 J/m2 UV light or not irradiated and were treated with 12.5 μM cycloheximide (CHX) for the indicated times. Cell lysates were analyzed by Western blotting with the indicated antibodies. The graph indicates average quantities of HBO1 proteins for three independent experiments. Error bars indicate standard errors (*, P < 0.05; **, P < 0.01). (D) UV-induced degradation was suppressed by the proteasome inhibitor. HEK293 cells were treated with 10 μM MG132 for 4 h and then irradiated with 100 J/m2 UV light or not irradiated. CHX (12.5 μM) was then added, and the cells were cultured for the indicated times. Cell lysates were analyzed by Western blotting with the indicated antibodies. The graph indicates average quantities of HBO1 proteins. Error bars indicate standard errors.
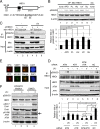
FIG 2
ATM/ATR-dependent phosphorylation of HBO1 at Ser50 and Ser53 is enhanced by DNA damage. (A) Schematic representation of the HBO1 protein. The amino acid sequence of HBO1 from residues 41 to 60 is shown, with the consensus sequences for ATM and ATR substrates underlined. (B) Various DNA damages and replication stresses induced phosphorylation at the Ser50 and Ser53 sites in HBO1. HEK293 cells overexpressing Myc-HBO1 WT were subjected to Western blotting with an anti-Ser50 and -Ser53 HBO1-specific antibody (pS50/53). Cells were exposed to genotoxic stresses for 1 h at the following concentrations: aphidicolin (APD), 50 μM; phleomycin (PL), 100 μM; hydroxyurea (HU), 2.5 mM; and bleomycin (BL), 40 μg/ml. The dose of UV light was 40 J/m2. Signal intensities were quantitated by use of ImageJ (**, P < 0.01). OP, overproduction. (C) Mutation of Ser50 and Ser53 in HBO1 to Ala abolished detection with the anti-pS50/53 antibody. Stably expressed HBO1 WT and S50/53A mutant proteins in HeLa shHBO1 cells and the original HeLa cells were irradiated with 40 J/m2 UV light. Cell lysates were prepared after 30 min of UV irradiation and subjected to IP-Western blotting with the indicated antibodies. (D) Phosphorylation of Ser50 and Ser53 of endogenous HBO1 was ATM and ATR dependent. ATM and/or ATR siRNAs were transfected into HeLa cells. At 48 h posttransfection, cells were exposed to 40 J/m2 UV light, cultured for 30 min, and then harvested. Cell lysates were analyzed by IP-Western blotting with the indicated antibodies. pS50/53 signals were quantitated by use of ImageJ (*, P < 0.05; **, P < 0.01; n.s., not significant). (E) Not only CPDs but also DSBs were generated by 40 J/m2 UV irradiation. Micropore UV irradiation was performed through an 8-μm micropore filter. CPDs and DSBs were detected indirectly by use of anti-XPC and anti-53BP1 antibodies, respectively. Accumulation of activated ATM at damage sites was detected by use of an anti-ATM pS1981 antibody. (F) Inhibition of ATM and ATR kinase activities suppressed phosphorylation of Ser50 and Ser53 in HBO1. Cells were incubated with ATM and ATR inhibitors for 2 h before UV irradiation at 40 J/m2. Cell lysates were prepared at 30 min post-UV treatment and analyzed by Western blotting with the indicated antibodies.
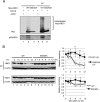
FIG 3
Phosphorylation of Ser50 and Ser53 of HBO1 after UV irradiation promotes ubiquitin-dependent degradation of HBO1. (A) Mutation of Ser50 and Ser53 to Ala suppressed HBO1 ubiquitylation after UV irradiation. HEK293 cells were transfected with Myc-HBO1 WT, Myc-HBO1 S50/53A, or empty vector together with HA-Ub, treated with MG132 for 4 h before UV irradiation at 40 J/m2, and then cultured for 3 h. Ubiquitylation of HBO1 was analyzed by Western blotting with the indicated antibodies. (B) Mutation of Ser50 and Ser53 to Ala protected HBO1 from degradation after UV irradiation. HEK293 cells were transfected with Myc-HBO1 WT or Myc-HBO1 S50/53A and irradiated with 100 J/m2 UV light or not irradiated. Cells were then treated with 12.5 μM CHX for the indicated times. The graphs indicate average HBO1 protein expression levels. Error bars indicate standard errors (*, P < 0.05; **, P < 0.01).
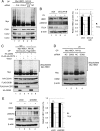
FIG 4
CRL4DDB2 ubiquitylates HBO1 in response to UV damage. (A) Depletion of CUL4A/B in HEK293 cells suppressed ubiquitylation of HBO1. CUL1, CUL2, and CUL4A/B were targeted by siRNA transfection for 48 h. Cell lysates were subjected to Western blotting with the indicated antibodies. (B) Depletion of CUL4A/B protected HBO1 phosphorylated at Ser50 and Ser53 from degradation after UV irradiation. CUL4A/B or negative-control siRNA was transfected into HEK293 cells. After 48 h of transfection, cells were exposed to 40 J/m2 UV light. Cells were incubated for 6 h, and Western blotting was performed with the indicated antibodies. β-Actin was used for normalization of HBO1 expression. The graph indicates the average HBO1 expression levels for three independent experiments. Error bars indicate standard errors (**, P < 0.01). (C) Overexpression of DDB2 induced ubiquitylation of HBO1 in vivo. Myc-HBO1, HA-Ub, Flag-DDB1, and HA-CUL4A were cotransfected with FLAG-HA-CDT2 or HA-DDB2 into HEK293 cells. Cells were treated with MG132 for 4 h before UV irradiation at 40 J/m2 and were then cultured for 2 h. Cell lysates were analyzed by Western blotting with the indicated antibodies. (D) Depletion of DDB2 suppressed ubiquitylation of HBO1. shCtl or shDDB2 cells were cotransfected with Myc-HBO1 and HA-Ub. Cells were treated with MG132 for 4 h before UV irradiation at 40 J/m2 and were then cultured for 6 h. (E) Depletion of DDB2 protected HBO1 phosphorylated at Ser50 and Ser53 from degradation after UV irradiation. shCtl or shDDB2 cells were exposed to 40 J/m2 UV light. Cells were incubated for 6 h, and Western blotting was performed with the indicated antibodies. β-Actin was used for normalization of HBO1 expression. The graph indicates the average HBO1 expression levels for three independent experiments. Error bars indicate standard errors (**, P < 0.01).
FIG 5
HBO1 interacts with DDB2 in response to UV damage. (A) Depletion of DDB2 suppressed degradation of HBO1 with or without UV treatment. shCtl or shDDB2 cells were exposed or not to 100 J/m2 UV light and treated with 12.5 μM CHX for the indicated times. β-Actin was used for normalization of HBO1 expression. The graphs indicate the average HBO1 expression levels for three independent experiments. Error bars indicate standard errors (**, P < 0.01). (B) The Myc-HBO1 WT but not S50/53A mutant protein interacted with HA-DDB2 after UV irradiation. HEK293 cells were transfected with Myc-HBO1 WT or S50/53A and HA-DDB2. Cells were irradiated with 40 J/m2 UV light and cultured for 30 min. Immunoprecipitation was performed with anti-HA antibody, and the immunoprecipitates were analyzed by immunoblotting with the indicated antibodies. (C) HA-DDB2 interacted with endogenous HBO1 after UV irradiation. HA-DDB2 was expressed in HEK293 cells. After 48 h of transfection, cells were exposed or not to 40 J/m2 UV light and then harvested 30 min after UV treatment. HA-DDB2 was immunoprecipitated by use of anti-HA antibody, and immunoprecipitates were subjected to immunoblotting with the indicated antibodies. (D) Endogenous HBO1 interacted with DDB2 after UV irradiation. HEK293 cells were irradiated with 40 J/m2 UV light and cultured for 10 min. Immunoprecipitation was performed with anti-DDB2 antibody, and the immunoprecipitates were analyzed by immunoblotting with the indicated antibodies.
FIG 6
HBO1 S50/53A cells fail to suppress cell proliferation and DNA repair and show increased apoptosis after UV irradiation. (A) HBO1 S50/53A cells failed to suppress proliferation after UV damage. Myc-HBO1 WT and S50/53A TG cells were irradiated with 8 and 15 J/m2 UV light, and cell numbers were counted 24 and 48 h after UV treatment. Two independent experiments were performed in triplicate with two independent clones and WT and S50/53A cells. Error bars indicate standard errors (**, P < 0.01). (B) HBO1 S50/53A cells failed to suppress acetylation of histone H3K14 after UV irradiation. Cell lysates of clone 1 from the experiments for panel A were analyzed for acetylation of histone H3K14, panacetylation of histone H4, and phosphorylation of Chk1 S317 by Western blotting with the indicated antibodies. Signals for histone H3 AcK14/total HH3, pan-acetyl histone H4/total HH4, and phosphorylated Chk1 S317/total Chk1 were quantitated by use of ImageJ. (C) HBO1 S50/53A cells were deficient in DNA repair after UV irradiation. The DNA repair efficiency in stable knockdown shHBO1/TG Myc-HBO1 WT or S50/53A cells was measured by use of alkaline comet assays. Error bars indicate standard errors (**, P < 0.01). (D) Cell cycle analysis of HBO1 WT- and S50/53A-expressing cells. HBO1 WT and S50/53A cells were irradiated with 15 J/m2 UV light and cultured for the indicated times. Cells were labeled with 20 μM BrdU for 40 min before harvest. Cells were fixed with 70% EtOH, and immunostaining was performed with anti-BrdU antibody. Total DNA content was measured by staining with propidium iodide. Cells were separated by FACS for cell cycle analysis. (E) HBO1 S50/53A cells showed increased apoptosis after UV damage. Myc-HBO1 WT and S50/53A TG cells were irradiated with 15 J/m2 UV light. Cells were incubated for 24 and 48 h after UV treatment. Apoptotic cells were detected with annexin V and analyzed by FACS. Error bars indicate standard errors (*, P < 0.05; **, P < 0.01).
Similar articles
- Deciphering structure, function and mechanism of lysine acetyltransferase HBO1 in protein acetylation, transcription regulation, DNA replication and its oncogenic properties in cancer.
Lan R, Wang Q. Lan R, et al. Cell Mol Life Sci. 2020 Feb;77(4):637-649. doi: 10.1007/s00018-019-03296-x. Epub 2019 Sep 18. Cell Mol Life Sci. 2020. PMID: 31535175 Free PMC article. Review. - SCF(Fbxw15) mediates histone acetyltransferase binding to origin recognition complex (HBO1) ubiquitin-proteasomal degradation to regulate cell proliferation.
Zou C, Chen Y, Smith RM, Snavely C, Li J, Coon TA, Chen BB, Zhao Y, Mallampalli RK. Zou C, et al. J Biol Chem. 2013 Mar 1;288(9):6306-16. doi: 10.1074/jbc.M112.426882. Epub 2013 Jan 14. J Biol Chem. 2013. PMID: 23319590 Free PMC article. - The scaffolding protein JADE1 physically links the acetyltransferase subunit HBO1 with its histone H3-H4 substrate.
Han J, Lachance C, Ricketts MD, McCullough CE, Gerace M, Black BE, Côté J, Marmorstein R. Han J, et al. J Biol Chem. 2018 Mar 23;293(12):4498-4509. doi: 10.1074/jbc.RA117.000677. Epub 2018 Jan 30. J Biol Chem. 2018. PMID: 29382722 Free PMC article. - Regulation of replication licensing by acetyltransferase Hbo1.
Iizuka M, Matsui T, Takisawa H, Smith MM. Iizuka M, et al. Mol Cell Biol. 2006 Feb;26(3):1098-108. doi: 10.1128/MCB.26.3.1098-1108.2006. Mol Cell Biol. 2006. PMID: 16428461 Free PMC article. - Multifunctional acyltransferase HBO1: a key regulatory factor for cellular functions.
Su Z, Zhang Y, Tang J, Zhou Y, Long C. Su Z, et al. Cell Mol Biol Lett. 2024 Nov 14;29(1):141. doi: 10.1186/s11658-024-00661-y. Cell Mol Biol Lett. 2024. PMID: 39543485 Free PMC article. Review.
Cited by
- BRPF3-HUWE1-mediated regulation of MYST2 is required for differentiation and cell-cycle progression in embryonic stem cells.
Cho HI, Kim MS, Lee J, Yoo BC, Kim KH, Choe KM, Jang YK. Cho HI, et al. Cell Death Differ. 2020 Dec;27(12):3273-3288. doi: 10.1038/s41418-020-0577-1. Epub 2020 Jun 18. Cell Death Differ. 2020. PMID: 32555450 Free PMC article. - SOX9 is targeted for proteasomal degradation by the E3 ligase FBW7 in response to DNA damage.
Hong X, Liu W, Song R, Shah JJ, Feng X, Tsang CK, Morgan KM, Bunting SF, Inuzuka H, Zheng XF, Shen Z, Sabaawy HE, Liu L, Pine SR. Hong X, et al. Nucleic Acids Res. 2016 Oct 14;44(18):8855-8869. doi: 10.1093/nar/gkw748. Epub 2016 Aug 26. Nucleic Acids Res. 2016. PMID: 27566146 Free PMC article. - Deciphering structure, function and mechanism of lysine acetyltransferase HBO1 in protein acetylation, transcription regulation, DNA replication and its oncogenic properties in cancer.
Lan R, Wang Q. Lan R, et al. Cell Mol Life Sci. 2020 Feb;77(4):637-649. doi: 10.1007/s00018-019-03296-x. Epub 2019 Sep 18. Cell Mol Life Sci. 2020. PMID: 31535175 Free PMC article. Review. - The emerging role for Cullin 4 family of E3 ligases in tumorigenesis.
Cheng J, Guo J, North BJ, Tao K, Zhou P, Wei W. Cheng J, et al. Biochim Biophys Acta Rev Cancer. 2019 Jan;1871(1):138-159. doi: 10.1016/j.bbcan.2018.11.007. Epub 2018 Dec 30. Biochim Biophys Acta Rev Cancer. 2019. PMID: 30602127 Free PMC article. Review. - Intrinsic ubiquitin E3 ligase activity of histone acetyltransferase Hbo1 for estrogen receptor α.
Iizuka M, Susa T, Tamamori-Adachi M, Okinaga H, Okazaki T. Iizuka M, et al. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):498-510. doi: 10.2183/pjab.93.030. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28769019 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous