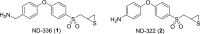Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy - PubMed (original) (raw)
Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy
Ming Gao et al. Proc Natl Acad Sci U S A. 2015.
Abstract
Nonhealing chronic wounds are major complications of diabetes resulting in >70,000 annual lower-limb amputations in the United States alone. The reasons the diabetic wound is recalcitrant to healing are not fully understood, and there are limited therapeutic agents that could accelerate or facilitate its repair. We previously identified two active forms of matrix metalloproteinases (MMPs), MMP-8 and MMP-9, in the wounds of db/db mice. We argued that the former might play a role in the body's response to wound healing and that the latter is the pathological consequence of the disease with detrimental effects. Here we demonstrate that the use of compound ND-336, a novel highly selective inhibitor of gelatinases (MMP-2 and MMP-9) and MMP-14, accelerates diabetic wound healing by lowering inflammation and by enhancing angiogenesis and re-epithelialization of the wound, thereby reversing the pathological condition. The detrimental role of MMP-9 in the pathology of diabetic wounds was confirmed further by the study of diabetic MMP-9-knockout mice, which exhibited wounds more prone to healing. Furthermore, topical administration of active recombinant MMP-8 also accelerated diabetic wound healing as a consequence of complete re-epithelialization, diminished inflammation, and enhanced angiogenesis. The combined topical application of ND-336 (a small molecule) and the active recombinant MMP-8 (an enzyme) enhanced healing even more, in a strategy that holds considerable promise in healing of diabetic wounds.
Keywords: MMP-8; MMP-9; ND-336; diabetic wound healing; inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Scheme 1.
Structures of compounds 1 and 2.
Fig. S1.
Synthesis of ND-336.
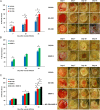
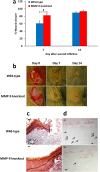
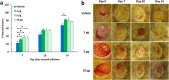
Fig. 1.
Effect of MMP-9 inhibition, topical treatment with exogenously added MMP-8, and combined MMP-9 inhibition and exogenous MMP-8 on diabetic wound healing. A single 8-mm wound was made on the dorsal thorax of db/db mice. *P < 0.05, #P < 0.01, &P < 0.001 indicate statistically significant differences in wound closure between the indicated groups. Statistical significance was evaluated by the two-tailed Mann–Whitney u test. (A) Wound healing after treatment with ND-336 (0.1 mg per wound per day), ND-322 (0.1 mg per wound per day) as a positive control, or vehicle. Data are shown as mean ± SEM (n = 8 mice per group on days 7, 10, and 14). (B) Wound healing after exogenously added MMP-8 (1 µg per wound per day). Data are shown as mean ± SEM (n = 20, 9, and 9 mice on days 7, 10, and 14, respectively, for the vehicle group; n = 20, 10, and 10 mice on days 7, 10, and 14, respectively, for the MMP-8 group). (C) Wound healing after treatment with combined ND-336 (0.05 mg per wound per day) and MMP-8 (1 µg per wound per day). Data are shown as mean ± SEM; n = 13, 14, 12, and 12 for the groups treated with vehicle, ND-336, MMP-8, and ND-336 + MMP-8, respectively.
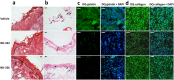
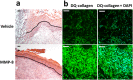
Fig. S2.
Effect of MMP-9 inhibition on diabetic wound healing. A single 8-mm wound was made on the dorsal thorax. Wounds were treated with ND-336 (0.1 mg per wound per day), ND-322 (0.1 mg per wound per day) as a positive control, or vehicle. H&E staining, TUNEL, and in situ zymography with DQ-gelatin and DQ-collagen were performed (n = 3 mice per group). (A) H&E staining on day 14. Re-epithelialization is indicated by the black line. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (B) TUNEL images of wounds on day 14. Arrows point to representative TUNEL+ (apoptotic) cells. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (C) In situ zymography with gelatinase fluorogenic substrate DQ-gelatin (Left, green), and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.) (D) In situ zymography with collagenase fluorogenic substrate DQ-collagen (Left, green) and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.)
Fig. S3.
Effect of MMP-9 gene ablation on diabetic wound healing. Diabetes was induced in wild-type and MMP-9–knockout mice by treatment with streptozotocin (150 mg/kg i.p.) and confirmed by a fasting blood glucose of >300 mg/dL. Excisional 8-mm wounds were inflicted 2 wk later. (A) Wound healing in wild-type and MMP-9–knockout streptozotocin-induced diabetic mice. Data are shown as mean ± SEM (n = 13 and 7 mice per group on days 7 and 14, respectively; total, 26 mice). #P < 0.01 indicates statistically significant differences in wound healing between the two indicated groups using the two-tailed Mann–Whitney u test. (B) Representative wound images (all to the same scale) on days 0, 7, and 14. (C) H&E staining for representative wounds in wild-type and MMP-9–knockout streptozotocin-induced diabetic mice (n = 3 mice per group per time point). Re-epithelialization is indicated by the black line. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (D) TUNEL images of representative wounds on day 7 (n = 3 mice per group per time point). Arrows point to representative TUNEL+ (apoptotic) cells. Pictures were taken with a 10× lens. (Scale bars, 50 µm.)
Fig. S4.
Topical treatment with exogenously added MMP-8 accelerates wound healing in db/db mice. A single 8-mm punch biopsy lesion on the dorsal thorax was administered to mice. Wounds were treated with MMP-8 (1 µg per wound per day; n = 20 mice) or vehicle (reaction buffer, n = 20 mice). H&E staining and in situ zymography with DQ-collagen were performed with on day 14 after wound infliction (n = 3 mice per group). (A) H&E staining for representative wounds treated with vehicle or MMP-8. Re-epithelialization is indicated by the black line. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (B) In situ zymography with collagenase fluorogenic substrate DQ-collagen (Left, green) and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.)
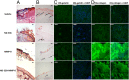
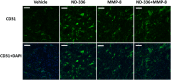
Fig. 2.
Effect of MMP-9 inhibition and exogenous MMP-8 on diabetic wound healing. Mice received a single 8-mm excisional wound on the dorsal thorax. Wounds were treated with vehicle, ND-336 (0.05 mg per wound per day), MMP-8 (1 µg per wound per day), or ND-336 (0.05 mg per wound per day) plus MMP-8 (1 µg per wound per day). H&E staining, TUNEL, and in situ zymography with DQ-gelatin and DQ-collagen were performed on day 14 (n = 3 mice per group). (A) H&E staining for representative wounds on day 14. Re-epithelialization is indicated by the black line. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (B) TUNEL images of representative wounds on day 14. Arrows point to representative TUNEL+ (apoptotic) cells. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (C) In situ zymography with gelatinase fluorogenic substrate DQ-gelatin (Left, green) and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.) (D) In situ zymography with collagenase fluorogenic substrate DQ-collagen (Left, green) and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.)
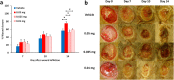
Fig. S5.
Dose–response of ND-336 in diabetic wound healing. (A) Wound healing in db/db mice treated with ND-336 at 0.05, 0.025, and 0.01 mg per wound per day. Data are shown as mean ± SEM (n = 7, 6, 7, and 7 mice, respectively, for vehicle, 0.05-mg, 0.025-mg, and 0.01-mg treatments; total, 27 mice). *P < 0.05 indicates statistically significant differences in wound healing by the two-tailed Mann–Whitney u test. (B) Representative wound images (all to the same scale) on days 0, 7, 10, and 14.
Fig. S6.
Dose–response of MMP-8 in diabetic wound healing. (A) Wound healing in db/db mice treated with MMP-8 at 1, 5, and 10 µg per wound per day. Data are shown as mean ± SEM (n = 20, 9, and 9 mice on days 7, 10, and 14, respectively, for the vehicle-treated group; n = 20, 10, and 10 mice on days 7, 10, and 14, respectively, for the group treated with 1 µg MMP-8 per wound per day; n = 10, 10, and 10 mice on days 7, 10, and 14, respectively, for the group treated with 5 µg MMP-8 per wound per day; n = 10, 10, and 9 mice on days 7, 10, and 14, respectively, for the group treated with 10 µg MMP-8 per wound per day). *P < 0.05 indicates statistically significant differences in wound healing using the two-tailed Mann–Whitney u test. (B) Representative wound images (all to the same scale) on days 0, 7, 10, and 14.
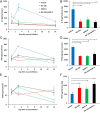
Fig. 3.
MMP-9 inhibition and/or exogenous MMP-8 result in decreased inflammation and increased angiogenesis. Data represent the mean ± SD (n = 3 mice per group per time point; total, 36 mice). *P < 0.05 and #P < 0.01 indicate statistically significant differences between the indicated groups. Statistical significance was evaluated by the Student’s t test using a two-tail distribution and unequal variance. (A) Concentrations of IL-6 as a function of time after wound infliction. (B) The AUC for IL-6 showed that IL-6 levels were reduced significantly upon treatment with ND-336, MMP-8, or combined ND-336 and MMP-8. (C) Concentrations of TGF-β1 as a function of time after wound infliction. (D) The AUC for TGF-β1 showed that TGF-β1 levels were reduced significantly upon treatment with ND-336, MMP-8, or combined ND-336 and MMP-8. (E) Concentrations of VEGF as a function of time after wound infliction. (F) AUC for VEGF showed that VEGF levels were increased significantly upon treatment with ND-336, MMP-8, or combined ND-336 and MMP-8.
Fig. S7.
MMP-9 inhibition, exogenous MMP-8 treatment, and combined MMP-9 inhibition and exogenous MMP-8 increase angiogenesis as measured by anti-CD31. Tissues were collected 14 d after wound infliction (n = 3 mice per group).
Similar articles
- Restructuring of the extracellular matrix in diabetic wounds and healing: A perspective.
Chang M. Chang M. Pharmacol Res. 2016 May;107:243-248. doi: 10.1016/j.phrs.2016.03.008. Epub 2016 Mar 24. Pharmacol Res. 2016. PMID: 27033051 - A chemical biological strategy to facilitate diabetic wound healing.
Gooyit M, Peng Z, Wolter WR, Pi H, Ding D, Hesek D, Lee M, Boggess B, Champion MM, Suckow MA, Mobashery S, Chang M. Gooyit M, et al. ACS Chem Biol. 2014 Jan 17;9(1):105-10. doi: 10.1021/cb4005468. Epub 2013 Sep 26. ACS Chem Biol. 2014. PMID: 24053680 Free PMC article. - Strategy for Treatment of Infected Diabetic Foot Ulcers.
Chang M, Nguyen TT. Chang M, et al. Acc Chem Res. 2021 Mar 2;54(5):1080-1093. doi: 10.1021/acs.accounts.0c00864. Epub 2021 Feb 17. Acc Chem Res. 2021. PMID: 33596041 Review. - Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing.
Nguyen TT, Ding D, Wolter WR, Pérez RL, Champion MM, Mahasenan KV, Hesek D, Lee M, Schroeder VA, Jones JI, Lastochkin E, Rose MK, Peterson CE, Suckow MA, Mobashery S, Chang M. Nguyen TT, et al. J Med Chem. 2018 Oct 11;61(19):8825-8837. doi: 10.1021/acs.jmedchem.8b01005. Epub 2018 Sep 27. J Med Chem. 2018. PMID: 30212201 - Matrix metalloproteinases: The sculptors of chronic cutaneous wounds.
Krishnaswamy VR, Mintz D, Sagi I. Krishnaswamy VR, et al. Biochim Biophys Acta Mol Cell Res. 2017 Nov;1864(11 Pt B):2220-2227. doi: 10.1016/j.bbamcr.2017.08.003. Epub 2017 Aug 7. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28797647 Review.
Cited by
- Immunomodulation in diabetic wounds healing: The intersection of macrophage reprogramming and immunotherapeutic hydrogels.
Sun D, Chang Q, Lu F. Sun D, et al. J Tissue Eng. 2024 Jul 27;15:20417314241265202. doi: 10.1177/20417314241265202. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 39071896 Free PMC article. Review. - Fluorinated Methacrylamide Chitosan Hydrogel Dressings Improve Regenerated Wound Tissue Quality in Diabetic Wound Healing.
Patil PS, Fathollahipour S, Inmann A, Pant A, Amini R, Shriver LP, Leipzig ND. Patil PS, et al. Adv Wound Care (New Rochelle). 2019 Aug 1;8(8):374-385. doi: 10.1089/wound.2018.0887. Epub 2019 Jul 25. Adv Wound Care (New Rochelle). 2019. PMID: 31346492 Free PMC article. - Assessment of Wound Healing Using MMP-8 Levels in GCF of Diabetics With Chronic Periodontitis After Diode Laser Assisted Flap Surgery.
Banerjee K, Gujjari SK, Madhunapantula SV. Banerjee K, et al. Acta Inform Med. 2023 Jun;31(2):126-130. doi: 10.5455/aim.2023.31.126-130. Acta Inform Med. 2023. PMID: 37711495 Free PMC article. - Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets.
Larouche J, Sheoran S, Maruyama K, Martino MM. Larouche J, et al. Adv Wound Care (New Rochelle). 2018 Jul 1;7(7):209-231. doi: 10.1089/wound.2017.0761. Adv Wound Care (New Rochelle). 2018. PMID: 29984112 Free PMC article. Review. - Static Magnetic Field Accelerates Diabetic Wound Healing by Facilitating Resolution of Inflammation.
Shang W, Chen G, Li Y, Zhuo Y, Wang Y, Fang Z, Yu Y, Ren H. Shang W, et al. J Diabetes Res. 2019 Nov 30;2019:5641271. doi: 10.1155/2019/5641271. eCollection 2019. J Diabetes Res. 2019. PMID: 31886281 Free PMC article.
References
- Centers for Disease Control and Prevenion 2014. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States. (Centers for Disease Control and Prevention, Atlanta, GA)
- Ziyadeh N, Fife D, Walker AM, Wilkinson GS, Seeger JD. A matched cohort study of the risk of cancer in users of becaplermin. Adv Skin Wound Care. 2011;24(1):31–39. - PubMed
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous