Sirt6 regulates dendritic cell differentiation, maturation, and function - PubMed (original) (raw)
doi: 10.18632/aging.100870.
Silvia Boero 2, Inga Bauer 1, Sara Morando 3, Patrizia Damonte 1, Michele Cea 1, Fiammetta Monacelli 1 2, Patrizio Odetti 1 2, Alberto Ballestrero 1 2, Antonio Uccelli 2 3, Raul Mostoslavsky 4, Alessandro Poggi 2, Alessio Nencioni 1 2
Affiliations
- PMID: 26761436
- PMCID: PMC4761712
- DOI: 10.18632/aging.100870
Sirt6 regulates dendritic cell differentiation, maturation, and function
Denise Lasigliè et al. Aging (Albany NY). 2016 Jan.
Abstract
Dendritic cells (DCs) are antigen-presenting cells that critically influence decisions about immune activation or tolerance. Impaired DC function is at the core of common chronic disorders and contributes to reduce immunocompetence during aging. Knowledge on the mechanisms regulating DC generation and function is necessary to understand the immune system and to prevent disease and immunosenescence. Here we show that the sirtuin Sirt6, which was previously linked to healthspan promotion, stimulates the development of myeloid, conventional DCs (cDCs). Sirt6-knockout (Sirt6KO) mice exhibit low frequencies of bone marrow cDC precursors and low yields of bone marrow-derived cDCs compared to wild-type (WT) animals. Sirt6KO cDCs express lower levels of class II MHC, of costimulatory molecules, and of the chemokine receptor CCR7, and are less immunostimulatory compared to WT cDCs. Similar effects in terms of differentiation and immunostimulatory capacity were observed in human monocyte-derived DCs in response to SIRT6 inhibition. Finally, while Sirt6KO cDCs show an overall reduction in their ability to produce IL-12, TNF-α and IL-6 secretion varies dependent on the stimulus, being reduced in response to CpG, but increased in response to other Toll-like receptor ligands. In conclusion, Sirt6 plays a crucial role in cDC differentiation and function and reduced Sirt6 activity may contribute to immunosenescence.
Keywords: Sirt6; TNF-α; Toll-like receptor ligands; costimulatory molecules; dendritic cells; immunosenescence.
Conflict of interest statement
Conflict of interest statement
The authors declare no competing financial interests.
Figures
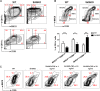
Figure 1. Sirt6 regulates the generation of cDCs in vivo and in vitro
(A, B) WT or Sirt6KO BM cells were cultured with GM-CSF and their ability to generate CD11c+ cells was analyzed at day 3, 5, 7 by flow cytometry. (A) The percentage of CD11c+ cells within cultures of Sirt6KO BMs (day 7) was normalized to that of WT CD11c+ cells. Results are presented are means ± SEM of 6 separate experiments, n=13 for each genotype; ***: p<0.001. (B) one representative experiment out of two is presented. (C-E) BM cells from WT and Sirt6KO mice were analyzed by flow cytometry for the frequency of cDC precursors (pre-cDCs, CD11c+MHCII−), pDCs, monocyte/macrophage subsets, mature granulocytes and of BM progenitors of different lineages. (C) one representative experiment out of six is presented; (D, E) results are presented are means ± SEM of fifteen and six separate experiments, respectively, n=6-15 for each genotype; *: p<0.05; **: p<0.01; n.s.: not significant. (F) TNF-α concentration in the supernatants of WT and Sirt6KO BMDCs (harvested at day 6) were determined by ELISA. Results are means ± SEM of three separate experiments, n=10 for each genotype; *: p<0.05. (G) WT and Sirt6KO BM cells were cultured with GM-SCF with or without addition of the indicated concentrations of TNF-α. CD11c+ cells were quantified at day 6 by flow cytometry. One representative experiment out of six is presented, n=6-9 for each genotype.
Figure 2. Sirt6 deletion hampers the spontaneous maturation of BMDCs
(A, B) BM cells from WT and Sirt6KO mice were cultured for 6 days with 20 ng/ml GM-CSF. At day 6, cells were re-seeded in the presence of 5 ng/ml GM-CSF and CD86, MHCII, and CD11c expression was determined at day 7 by flow cytometry. (A, B) One representative experiment out of eight is presented. (B) lower inset, results are means ± SEM of eight separate experiments; n=13 for each genotype; *: p<0.05; **: p<0.01. (C) WT and Sirt6KO BMDCs harvested at day 6 of culture were re-seeded with TNF-α at the indicated concentrations. 24 h later, BMDCs were harvested and analyzed by flow cytometry. One representative experiment out of five is presented (n=8 for each genotype).
Figure 3. In vitro generated Sirt6KO BMDCs show increased endocytic activity and impaired allostimulatory capacity
(A, B) WT and Sirt6KO BMDCs were harvested at day 7 and incubated with dextran-FITC for 30 min at 37°C or at 4°C. Thereafter, cells were stained for CD11c and finally analyzed by flow cytometry. (A) Gating was done on CD11c+ cells; one representative experiment out of six is presented. (B) Dextran-FITC+/CD11c+ Sirt6KO BMDCs were enumerated and their frequency was normalized to that of dextran-FITC+/CD11c+ WT BMDCs. Results are means ± SEM of six separate experiments, n=6 for each genotype. (C) purified allogeneic (BALB-c) CD4+ splenocytes (responders) were stained with CFSE and incubated with 5 μg/ml phythoemagglutinin (PHA) or with sorted, WT or Sirt6KO, CD11c+MHCII+ BMDCs at the indicated S:R ratios. Proliferation of alive (propidium-iodide negative) CD3+CD4+CD11c− cells was evaluated by carboxyfluorescein succinimidyl ester (CFSE) dilution after a 5-day incubation. One representative experiment out of three is presented, n=4 for each genotype.
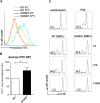
Figure 4. Sirt6KO BMDCs show impaired maturation and CCR7 expression in response to TLR ligands
(A-C) WT and Sirt6KO BMDCs were harvested at day 7 and stimulated with or without LPS or with CpG for 24 h. Thereafter, cells were harvested, washed, and MHCII, CD86, CD80, CD40 and CCR7 expression on CD11c+ cells was analyzed by flow cytometry. (A, B) One representative experiment out of ten (A) or out of four (B) is presented (n=4-21 for each genotype). (C) CCR7 mean fluorescence intensity (MFI) was normalized to that of WT BMDCs. Results are means ± SEM of four separate experiments, n=11 for each genotype; ***: p<0.001. (D) WT and Sirt6KO BMDCs were harvested at day 7 and stimulated for 24 h with LPS. Thereafter, cell migration to CCL21 was evaluated. The percentage of Sirt6KO BMDCs that had migrated was normalized to that of WT BMDCs. Results are means ± SEM of three separate experiments, n=4 for each genotype; *: p<0.05.
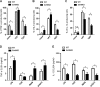
Figure 5. Sirt6 deletion skews cytokine production in BMDCs
(A-E) WT and Sirt6KO BMDCs were harvested at day 8 and stimulated for 24 h with or without different TLR ligands. (A-C) Cells were harvested, stained for CD11c, CD86 and intracellular TNF-α, IL-6 or IL-12. CD11c+CD86+ TNF-α-, IL-6-, or IL-12-producing cells were quantified by flow cytometry. Results are means ± SEM of three-to-five separate experiments, n=3-10 for each genotype. In (D, E), cytokine secretion into cell supernatants was measured by ELISA. Results are means ± SEM of 3-10 separate experiments; *: p<0.05; n.s.: not significant.
Figure 6. SIRT6 inhibition prevents moDC differentiation
(A-C) Human adherent PBMCs were cultured with GM-CSF and IL-4 for 6 days in the presence of either S6 (at the indicated concentrations) or vehicle DMSO. Thereafter, cells were harvested, washed and analyzed by flow cytometry for CD14 and CD1a expression or utilized as stimulators in MLR. (A) One representative experiment out of five is presented. (B, C) Results are means ± SEM of those obtained with five different donors. *: p<0.05; ***: p<0.001.
Similar articles
- Immunomodulation by Trypanosoma cruzi: toward understanding the association of dendritic cells with infecting TcI and TcII populations.
da Costa TA, Silva MV, Mendes MT, Carvalho-Costa TM, Batista LR, Lages-Silva E, Rodrigues V, Oliveira CJ, Ramirez LE. da Costa TA, et al. J Immunol Res. 2014;2014:962047. doi: 10.1155/2014/962047. Epub 2014 Oct 13. J Immunol Res. 2014. PMID: 25371910 Free PMC article. - Bisphenol A Does Not Mimic Estrogen in the Promotion of the In Vitro Response of Murine Dendritic Cells to Toll-Like Receptor Ligands.
Chakhtoura M, Sriram U, Heayn M, Wonsidler J, Doyle C, Dinnall JA, Gallucci S, Roberts RA. Chakhtoura M, et al. Mediators Inflamm. 2017;2017:2034348. doi: 10.1155/2017/2034348. Epub 2017 Jul 25. Mediators Inflamm. 2017. PMID: 28811679 Free PMC article. - Bromelain treatment leads to maturation of monocyte-derived dendritic cells but cannot replace PGE2 in a cocktail of IL-1β, IL-6, TNF-α and PGE2.
Karlsen M, Hovden AO, Vogelsang P, Tysnes BB, Appel S. Karlsen M, et al. Scand J Immunol. 2011 Aug;74(2):135-43. doi: 10.1111/j.1365-3083.2011.02562.x. Scand J Immunol. 2011. PMID: 21449940 - Beyond CCR7: dendritic cell migration in type 2 inflammation.
Meloun A, León B. Meloun A, et al. Front Immunol. 2025 Feb 28;16:1558228. doi: 10.3389/fimmu.2025.1558228. eCollection 2025. Front Immunol. 2025. PMID: 40093008 Free PMC article. Review. - Sirtuin 6, a possible therapeutic target for type 2 diabetes.
Bae EJ. Bae EJ. Arch Pharm Res. 2017 Dec;40(12):1380-1389. doi: 10.1007/s12272-017-0989-8. Epub 2017 Nov 25. Arch Pharm Res. 2017. PMID: 29177584 Review.
Cited by
- Research progress of SIRTs activator resveratrol and its derivatives in autoimmune diseases.
Yu X, Chen M, Wu J, Song R. Yu X, et al. Front Immunol. 2024 Jun 19;15:1390907. doi: 10.3389/fimmu.2024.1390907. eCollection 2024. Front Immunol. 2024. PMID: 38962006 Free PMC article. Review. - Sirtuin1 Targeting Reverses Innate and Adaptive Immune Tolerance in Septic Mice.
Martin AN, Alexander-Miller M, Yoza BK, Vachharajani V, McCall CE. Martin AN, et al. J Immunol Res. 2018 Jul 4;2018:2402593. doi: 10.1155/2018/2402593. eCollection 2018. J Immunol Res. 2018. PMID: 30069485 Free PMC article. - Role of Sirtuins in Physiology and Diseases of the Central Nervous System.
Chojdak-Łukasiewicz J, Bizoń A, Waliszewska-Prosół M, Piwowar A, Budrewicz S, Pokryszko-Dragan A. Chojdak-Łukasiewicz J, et al. Biomedicines. 2022 Sep 29;10(10):2434. doi: 10.3390/biomedicines10102434. Biomedicines. 2022. PMID: 36289696 Free PMC article. Review. - Sirt6 inhibition delays the onset of experimental autoimmune encephalomyelitis by reducing dendritic cell migration.
Ferrara G, Benzi A, Sturla L, Marubbi D, Frumento D, Spinelli S, Abbotto E, Ivaldi F, von Holtey M, Murone M, Nencioni A, Uccelli A, Bruzzone S. Ferrara G, et al. J Neuroinflammation. 2020 Jul 31;17(1):228. doi: 10.1186/s12974-020-01906-1. J Neuroinflammation. 2020. PMID: 32736564 Free PMC article. - Sirtuins at the Service of Healthy Longevity.
Watroba M, Szukiewicz D. Watroba M, et al. Front Physiol. 2021 Nov 25;12:724506. doi: 10.3389/fphys.2021.724506. eCollection 2021. Front Physiol. 2021. PMID: 34899370 Free PMC article. Review.
References
- Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clinical immunology. 2007;122:220–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials