Myocardial KChIP2 Expression in Guinea Pig Resolves an Expanded Electrophysiologic Role - PubMed (original) (raw)
Myocardial KChIP2 Expression in Guinea Pig Resolves an Expanded Electrophysiologic Role
Drew M Nassal et al. PLoS One. 2016.
Abstract
Cardiac ion channels and their respective accessory subunits are critical in maintaining proper electrical activity of the heart. Studies have indicated that the K+ channel interacting protein 2 (KChIP2), originally identified as an auxiliary subunit for the channel Kv4, a component of the transient outward K+ channel (Ito), is a Ca2+ binding protein whose regulatory function does not appear restricted to Kv4 modulation. Indeed, the guinea pig myocardium does not express Kv4, yet we show that it still maintains expression of KChIP2, suggesting roles for KChIP2 beyond this canonical auxiliary interaction with Kv4 to modulate Ito. In this study, we capitalize on the guinea pig as a system for investigating how KChIP2 influences the cardiac action potential, independent of effects otherwise attributed to Ito, given the endogenous absence of the current in this species. By performing whole cell patch clamp recordings on isolated adult guinea pig myocytes, we observe that knock down of KChIP2 significantly prolongs the cardiac action potential. This prolongation was not attributed to compromised repolarizing currents, as IKr and IKs were unchanged, but was the result of enhanced L-type Ca2+ current due to an increase in Cav1.2 protein. In addition, cells with reduced KChIP2 also displayed lowered INa from reduced Nav1.5 protein. Historically, rodent models have been used to investigate the role of KChIP2, where dramatic changes to the primary repolarizing current Ito may mask more subtle effects of KChIP2. Evaluation in the guinea pig where Ito is absent, has unveiled additional functions for KChIP2 beyond its canonical regulation of Ito, which defines KChIP2 as a master regulator of cardiac repolarization and depolarization.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Fig 1. Conservation and expression of KChIP2 across species.
(A) Protein sequence alignment across multiple mammals shows a high degree of sequence homology for KChIP2. (B) Relative expression of kcnip2 (KChIP2) mRNA isolated from ventricular tissue (presented as means ± SEM, n = 3, guinea pig n = 5). Guinea pig expression is significantly less than other species but comparable to neonatal rat tissue where I to is present.
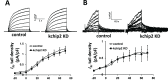
Fig 2. KChIP2 knockdown in isolated ventricular myocytes of the guinea pig prolongs the cardiac action potential.
(A) Immunoblot for KChIP2 of whole cell lysate isolated from ventricular cells treated for 24 hrs with adenovirus encoding either GFP (control) or an mRNA antisense sequence for KChIP2 (KChIP2 KD). Beta-actin was used as a loading control (B) Representative action potential tracings at 1000 ms cycle length from isolated ventricular cells following 24 hrs incubation with Ad.KChIP2-KD. (C) Summary data of APD90 during 1000 ms and 400 ms cycle lengths. KChIP2 KD cells at 1000 ms (n = 16) show a significant prolongation of APD, compared to control cells (n = 14). KChIP2 KD at 400 ms (n = 11) was also significantly prolonged, compared to control cells (n = 9). (D) APD50 following 1000 ms and 400 ms cycle lengths for the same treatment groups. Data presented as mean ± SEM; *P < 0.05; two-tailed Student’s _t_-test.
Fig 3. Action potential parameters.
(A) Action potential amplitude and (B) resting membrane potential between control (n = 14) and Ad.KChIP2-KD (n = 16) isolated cardiomyocytes at 24 hrs shows no significant difference. (C) Left panel shows representative peak I K1 elicited by a voltage step to -100 mV from a holding potential of -40 mV. Right panel shows summary data of these peak averages, indicating no significant change between control (n = 11) and KChIP2 KD (n = 10) cardiomyocytes. Data presented as mean ± SEM; two-tailed Student’s _t_-test performed.
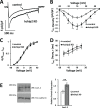
Fig 4. APD prolongation observed following KChIP2 silencing is not defined by compromised repolarization.
(A) Upper panel shows representative current traces for I Ks for both control and KChIP2 KD cardiomyocytes following 24 hrs incubation. Currents were generated from a holding potential of -40 mV with depolarizing voltage pulses from -30 mV to 60 mV, and then a return to -40 mV to generate outward tail currents in the presence of E4031. Lower panel shows the resulting I/V curve summary data between control (n = 19) and KChIP2 KD (n = 10) yielding no significant difference between treatment groups. (B) Upper panel shows representative current traces for I Kr for both control and KChIP2 KD cardiomyocytes following 24 hrs incubation. Currents were isolated as E4031 sensitive. Lower panel shows the resulting I/V curve summary data for tail currents between control (n = 9) and KChIP2 KD (n = 4). There was no significant difference between the two groups. Data presented as mean ± SEM; two-tailed Student’s _t_-test performed.
Fig 5. KChIP2 knock down enhances density of L-type Ca2+ current.
(A) Representative I Ca,L current traces of control and KChIP2 KD treated cells following 24 hrs incubation. Currents were elicited from a holding potential of -40 mV and a depolarizing step to 10 mV. (B) I/V curve summary data of I Ca,L displays significant enhancement of current density following KChIP2 KD (n = 12) compared to control cardiomyocytes (n = 20), explaining the prolonged plateau phase of the cardiac action potential. (C) Steady-state activation of I Ca,L in control and KChIP2 KD treated myocytes. The data, depicted as normalized conductance, was fit with a Boltzmann curve but no significant difference was detected at any test pulse. (D) Evaluation of current decay kinetics between control and KChIP2 KD treated myocytes at several membrane voltages. _τ_-values were derived from single exponential fits of I Ca,L decay following channel activation, also showing no significant difference at any test pulses. (E) Left panel shows representative immunoblots for Cav1.2 protein from whole cell lysates for control and KChIP2 KD treated myocytes. Protein expression was normalized to pan-cadherin expression. Right panel shows summary data depicting the normalized, relative densitometry of Cav1.2 expression, resulting in significantly increased expression following KChIP2 KD (n = 4). Data presented as mean ± SEM; *P < 0.05, **P < 0.01; two-tailed Student’s _t_-test performed for I/V plot, paired two-tailed Student’s _t_-test performed for Western blot.
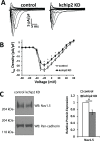
Fig 6. KChIP2 knock down attenuates I Na density.
(A) Representative traces for I Na in cardiomyocytes in control and KChIP2 KD cells following 24 hrs incubation. (B) Summary data of the I/V curve for control (n = 13) and KChIP2 KD (n = 12) cardiomyocytes, showing reduced current density in response to KChIP2 loss. (C) Left panel shows representative immunoblots for Nav1.5 protein from whole cell lysates for control and KChIP2 KD treated myocytes. Protein expression was normalized to pan-cadherin expression. Right panel shows the average fold change from KChIP2 KD treated cardiomyocytes from control, which resulted in significantly decreased expression following KChIP2 KD (n = 3). Data presented as mean ± SEM; *P < 0.05 two-tailed Student’s _t_-test performed for I/V plot, paired two-tailed Student’s _t_-test performed for Western blot.
Similar articles
- Stabilization of Kv4 protein by the accessory K(+) channel interacting protein 2 (KChIP2) subunit is required for the generation of native myocardial fast transient outward K(+) currents.
Foeger NC, Wang W, Mellor RL, Nerbonne JM. Foeger NC, et al. J Physiol. 2013 Sep 1;591(17):4149-66. doi: 10.1113/jphysiol.2013.255836. Epub 2013 May 27. J Physiol. 2013. PMID: 23713033 Free PMC article. - Prolonged leptin treatment increases transient outward K⁺ current via upregulation of Kv4.2 and Kv4.3 channel subunits in adult rat ventricular myocytes.
Gómez-Hurtado N, Fernández-Velasco M, Fernández-Alfonso MS, Boscá L, Delgado C. Gómez-Hurtado N, et al. Pflugers Arch. 2014 May;466(5):903-14. doi: 10.1007/s00424-013-1348-3. Epub 2013 Sep 18. Pflugers Arch. 2014. PMID: 24046152 - Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2.
Sato T, Kobayashi T, Kuno A, Miki T, Tanno M, Kouzu H, Itoh T, Ishikawa S, Kojima T, Miura T, Tohse N. Sato T, et al. Am J Physiol Heart Circ Physiol. 2014 Apr 1;306(7):H1054-65. doi: 10.1152/ajpheart.00414.2013. Epub 2014 Jan 31. Am J Physiol Heart Circ Physiol. 2014. PMID: 24486512 - KV Channel-Interacting Proteins in the Neurological and Cardiovascular Systems: An Updated Review.
Wu LY, Song YJ, Zhang CL, Liu J. Wu LY, et al. Cells. 2023 Jul 20;12(14):1894. doi: 10.3390/cells12141894. Cells. 2023. PMID: 37508558 Free PMC article. Review. - Proteomic analysis highlights the molecular complexities of native Kv4 channel macromolecular complexes.
Marionneau C, Townsend RR, Nerbonne JM. Marionneau C, et al. Semin Cell Dev Biol. 2011 Apr;22(2):145-52. doi: 10.1016/j.semcdb.2010.10.004. Epub 2010 Oct 17. Semin Cell Dev Biol. 2011. PMID: 20959143 Free PMC article. Review.
Cited by
- Effect of Transmural Differences in Excitation-Contraction Delay and Contraction Velocity on Left Ventricle Isovolumic Contraction: A Simulation Study.
Vaverka J, Burša J, Šumbera J, Pásek M. Vaverka J, et al. Biomed Res Int. 2018 May 10;2018:4798512. doi: 10.1155/2018/4798512. eCollection 2018. Biomed Res Int. 2018. PMID: 29862273 Free PMC article. - Notch signaling modulates the electrical behavior of cardiomyocytes.
Borghetti G, Eisenberg CA, Signore S, Sorrentino A, Kaur K, Andrade-Vicenty A, Edwards JG, Nerkar M, Qanud K, Sun D, Goichberg P, Leri A, Anversa P, Eisenberg LM, Jacobson JT, Hintze TH, Rota M. Borghetti G, et al. Am J Physiol Heart Circ Physiol. 2018 Jan 1;314(1):H68-H81. doi: 10.1152/ajpheart.00587.2016. Epub 2017 Sep 22. Am J Physiol Heart Circ Physiol. 2018. PMID: 28939651 Free PMC article. - KChIP2 regulates the cardiac Ca2+ transient and myocyte contractility by targeting ryanodine receptor activity.
Nassal DM, Wan X, Liu H, Laurita KR, Deschênes I. Nassal DM, et al. PLoS One. 2017 Apr 6;12(4):e0175221. doi: 10.1371/journal.pone.0175221. eCollection 2017. PLoS One. 2017. PMID: 28384221 Free PMC article. - The adhesion function of the sodium channel beta subunit (β1) contributes to cardiac action potential propagation.
Veeraraghavan R, Hoeker GS, Alvarez-Laviada A, Hoagland D, Wan X, King DR, Sanchez-Alonso J, Chen C, Jourdan J, Isom LL, Deschenes I, Smyth JW, Gorelik J, Poelzing S, Gourdie RG. Veeraraghavan R, et al. Elife. 2018 Aug 14;7:e37610. doi: 10.7554/eLife.37610. Elife. 2018. PMID: 30106376 Free PMC article. - Potassium Channel Interacting Protein 2 (KChIP2) is not a transcriptional regulator of cardiac electrical remodeling.
Winther SV, Tuomainen T, Borup R, Tavi P, Antoons G, Thomsen MB. Winther SV, et al. Sci Rep. 2016 Jun 28;6:28760. doi: 10.1038/srep28760. Sci Rep. 2016. PMID: 27349185 Free PMC article.
References
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403(6769):553–6. - PubMed
- An RH, Wang XL, Kerem B, Benhorin J, Medina A, Goldmit M, et al. Novel LQT-3 mutation affects Na+ channel activity through interactions between alpha- and beta1-subunits. Circ Res. 1998;83(2):141–6. - PubMed
- Bahring R, Dannenberg J, Peters HC, Leicher T, Pongs O, Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel- interacting protein 2.2 on channel expression and gating. The Journal of biological chemistry. 2001;276(26):23888–94. - PubMed
- Decher N, Uyguner O, Scherer CR, Karaman B, Yuksel-Apak M, Busch AE, et al. hKChIP2 is a functional modifier of hKv4.3 potassium channels: cloning and expression of a short hKChIP2 splice variant. Cardiovasc Res. 2001;52(2):255–64. - PubMed
- Deschenes I, DiSilvestre D, Juang GJ, Wu RC, An WF, Tomaselli GF. Regulation of Kv4.3 current by KChIP2 splice variants: a component of native cardiac I(to)? Circulation. 2002;106(4):423–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL096962/HL/NHLBI NIH HHS/United States
- T32 HL105338/HL/NHLBI NIH HHS/United States
- T32 HL105338-01/HL/NHLBI NIH HHS/United States
- R01HL096962)/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous