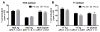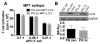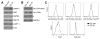LAMP-2C Inhibits MHC Class II Presentation of Cytoplasmic Antigens by Disrupting Chaperone-Mediated Autophagy - PubMed (original) (raw)
LAMP-2C Inhibits MHC Class II Presentation of Cytoplasmic Antigens by Disrupting Chaperone-Mediated Autophagy
Liliana Pérez et al. J Immunol. 2016.
Abstract
Cells use multiple autophagy pathways to sequester macromolecules, senescent organelles, and pathogens. Several conserved isoforms of the lysosome-associated membrane protein-2 (LAMP-2) regulate these pathways influencing immune recognition and responses. LAMP-2A is required for chaperone-mediated autophagy (CMA), which promotes Ag capture and MHC class II (MHCII) presentation in B cells and signaling in T cells. LAMP-2B regulates lysosome maturation to impact macroautophagy and phagocytosis. Yet, far less is known about LAMP-2C function. Whereas LAMP2A and LAMP2B mRNA were broadly detected in human tissues, LAMP2C expression was more limited. Transcripts for the three LAMP2 isoforms increased with B cell activation, although specific gene induction varied depending on TLR versus BCR engagement. To examine LAMP-2C function in human B cells and specifically its role in Ag presentation, we used ectopic gene expression. Increased LAMP-2C expression in B cells did not alter MHCII expression or invariant chain processing, but did perturb cytoplasmic Ag presentation via CMA. MHCII presentation of epitopes from exogenous and membrane Ags was not affected by LAMP-2C expression in B cells. Similarly, changes in B cell LAMP-2C expression did not impact macroautophagy. The gene expression of other LAMP2 isoforms and proteasome and lysosomal proteases activities were unperturbed by LAMP-2C ectopic expression. LAMP-2C levels modulated the steady-state expression of several cytoplasmic proteins that are targeted for degradation by CMA and diminished peptide translocation via this pathway. Thus, LAMP-2C serves as a natural inhibitor of CMA that can selectively skew MHCII presentation of cytoplasmic Ags.
Copyright © 2016 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
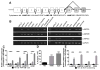
Figure 1. LAMP2 isoform expression and regulation during B cell activation
(A) Exon structure and alternative splicing of human LAMP2. The three isoforms have an identical luminal domain but distinct transmembrane and cytoplasmic domains. (B) RT-PCR analysis for LAMP2 isoforms in human tissues. LAMP2A and LAMP2B are ubiquitously expressed while LAMP2C is tissue specific. (C) Peripheral blood human B cells were treated for 24 h with R848, CpG, or left untreated (NS). Gene expression of LAMP2 isoforms and CD86 were analyzed by qPCR. (D) B cell TLR7 or TLR9 stimulation was detected via IL-6 release. (E) Peripheral blood B cells were stimulated for 24 h to crosslink BCR or left untreated (NS). LAMP2 isoforms and CD86 mRNA were detected as in (C). Data were analyzed by two-way ANOVA (C and E) or by one-way ANOVA (D). *p < 0.05, ***p < 0.001, and ****p < 0.0001 (mean ± SD, n = 3).
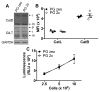
Figure 2. Inhibition of MHCII cytoplasmic Ag presentation in B cells ectopically expressing LAMP-2C
(A) PG zeo and PG 2c B cells with ectopic LAMP-2C were incubated with GAD-specific T cells to analyze T cell activation. (B) As a control, PG zeo and PG 2c B cells were incubated with 10 μM of GAD273-285 peptide for 4 h and cultured with GAD-specific T cells at APC:T cell ratio of 0.05:1 to monitor MHCII presentation. (C) FS pCMV and FS 2c B cells were incubated with κ-specific T cells at APC:T cell ratio of 1:1 to detect T cell activation. (D) FS pCMV and FS 2c B cells were incubated overnight with 10 μM of κ188-203 peptide and cultured with κ-specific T cells at APC:T cell ratio of 1:1 to measure T cell activation. Data were analyzed by two-way ANOVA (A) or by two-tailed, unpaired Student t test (C). **p < 0.01 and ***p < 0.001 (mean ± SD, representative of n 3).
Figure 3. LAMP-2C expression did not alter the MHCII presentation of exogenous Ags
(A) B cells +/− ectopic LAMP-2C expression were incubated overnight with 20 μM of HSA Ag or 4 h with 10 μM HSA64-76 peptide and then cultured with HSA-specific T cells to measure T cell activation. (B) PG zeo and PG 2c B cells were incubated overnight with 0.1 μM of TT Ag or for 4 h with 0.2 μM of TT peptides. APCs were cultured with TT-specific T cells to analyze T cell activation. (mean ± SD, representative of n 3).
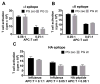
Figure 4. MHCII presentation of self or viral membrane proteins was not affected by LAMP-2C overexpression
(A and B) PG zeo and PG 2c B cells were cultured with κI- or κII-specific T cells. T cell activation was monitored to analyze MHCII presentation of membrane Ag, Ig κ. (C) PG zeo and PG 2c B cells were infected overnight with influenza A X-31, A/Aichi/68 (H3N2) or incubated with HA307-319 peptide for 4 h and then cultured with HA-specific T cells to measure T cell activation. (mean ± SD, representative of n 3).
Figure 5. Macroautophagy was not altered with B cell ectopic LAMP-2C expression
(A) PG zeo and PG 2c B cells were transduced to express the chimeric Ag MP1-LC3. MP1-LC3 is targeted to autophagosomes by the LC3 domain. APCs were cultured with MP1-specific T cells to monitor T cell activation. (B) PG zeo and PG 2c B cells were incubated overnight +/− 20 μM CQ, an inhibitor of lysosome acidification, to monitor autophagosome formation and turnover. MA flux was evaluated by immunoblotting to detect changes in cellular LC3II levels +/− CQ. (mean ± SD, representative of n = 2–3).
Figure 6. Changes in cytoplasmic MHCII-restricted presentation were not due to differential expression of proteins required for Ag presentation or CMA
(A and B) Lysates from PG zeo and PG 2c B cells were resolved by SDS-PAGE and immunoblotted to detect LAMP-2, GAD, HSC70, HSP90, LAMP-1, HLA-DR dimer, HLA-DR α chain, Ii, actin, and GAPDH. (C) PG zeo and PG 2c B cells were incubated with Abs to detect cell surface expression of HLA-DR, HLA-DQ, HLA-DP, or CLIP and total cellular levels of HLA-DO. (mean ± SD, representative of n 4).
Figure 7. Cellular lysosomal enzyme levels and proteolytic processing by cathepsins and the proteasome were unaffected by ectopic expression of LAMP-2C in B cells
(A) Comparable maturation of CatD and GILT in B cells expressing LAMP-2C. Lysates from PG zeo and PG 2c B cells were resolved by SDS-PAGE and immunoblotted to detect the precursor (p), intermediate (i) or mature (m) form of CatD or GILT. (B) To analyze CatL or CatB activities, PG zeo and PG 2c B cells were incubated for 30 min at 37°C with membrane permeable fluorogenic substrates specific for CatL or CatB. Enzyme activity was detected by flow cytometry. (C) Proteasome activity was determined by incubating PG zeo and PG 2c B cells with a luminogenic chymotrypsin-like substrate. (mean ± SD, representative of n = 2–4).
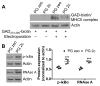
Figure 8. Decreased peptide translocation from the cytoplasm to endosomal MHCII molecules and reduced processing of CMA substrates
(A) PG zeo and PG 2c B cells were incubated with 2 mM GAD273-285-biotin and electroporated to deliver this peptide to the cytoplasm. Control cells were not subjected to electroporation. Cells were acid- stripped, cultured 16 h, and lysates resolved by SDS-PAGE. Streptavidin-HRP was used to detect biotin-peptide complexed with MHCII molecules. Immunoblotting of actin was used as a loading control. (B) Basal levels of CMA substrates p-IκBα and RNAse A were evaluated by immunoblotting. Data were analyzed by two-way ANOVA. ***p < 0.001 (mean ± SD, n = 3–4).
Similar articles
- Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens.
Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Zhou D, et al. Immunity. 2005 May;22(5):571-81. doi: 10.1016/j.immuni.2005.03.009. Immunity. 2005. PMID: 15894275 - LAMP-2-deficient human B cells exhibit altered MHC class II presentation of exogenous antigens.
Crotzer VL, Glosson N, Zhou D, Nishino I, Blum JS. Crotzer VL, et al. Immunology. 2010 Nov;131(3):318-30. doi: 10.1111/j.1365-2567.2010.03309.x. Immunology. 2010. PMID: 20518820 Free PMC article. - Modulation of deregulated chaperone-mediated autophagy by a phosphopeptide.
Macri C, Wang F, Tasset I, Schall N, Page N, Briand JP, Cuervo AM, Muller S. Macri C, et al. Autophagy. 2015;11(3):472-86. doi: 10.1080/15548627.2015.1017179. Autophagy. 2015. PMID: 25719862 Free PMC article. - LAMP2A, LAMP2B and LAMP2C: similar structures, divergent roles.
Qiao L, Hu J, Qiu X, Wang C, Peng J, Zhang C, Zhang M, Lu H, Chen W. Qiao L, et al. Autophagy. 2023 Nov;19(11):2837-2852. doi: 10.1080/15548627.2023.2235196. Epub 2023 Jul 21. Autophagy. 2023. PMID: 37469132 Free PMC article. Review. - Cytosol to lysosome transport of intracellular antigens during immune surveillance.
Crotzer VL, Blum JS. Crotzer VL, et al. Traffic. 2008 Jan;9(1):10-6. doi: 10.1111/j.1600-0854.2007.00664.x. Epub 2007 Oct 31. Traffic. 2008. PMID: 17916226 Free PMC article. Review.
Cited by
- Altered Splicing of LAMP2 in a Multigenerational Family from Latvia Affected by Danon Disease.
Stavusis J, Micule I, Grinfelde I, Zdanovica A, Pudulis J, Valeina S, Sepetiene S, Lace B, Inashkina I. Stavusis J, et al. Medicina (Kaunas). 2024 Jan 5;60(1):99. doi: 10.3390/medicina60010099. Medicina (Kaunas). 2024. PMID: 38256360 Free PMC article. - Oxidation inhibits autophagy protein deconjugation from phagosomes to sustain MHC class II restricted antigen presentation.
Ligeon LA, Pena-Francesch M, Vanoaica LD, Núñez NG, Talwar D, Dick TP, Münz C. Ligeon LA, et al. Nat Commun. 2021 Mar 8;12(1):1508. doi: 10.1038/s41467-021-21829-6. Nat Commun. 2021. PMID: 33686057 Free PMC article. - Lysosome-Associated Membrane Proteins Support the Furin-Mediated Processing of the Mumps Virus Fusion Protein.
Ueo A, Kubota M, Shirogane Y, Ohno S, Hashiguchi T, Yanagi Y. Ueo A, et al. J Virol. 2020 Jun 1;94(12):e00050-20. doi: 10.1128/JVI.00050-20. Print 2020 Jun 1. J Virol. 2020. PMID: 32295904 Free PMC article. - Chaperone-Mediated Autophagy in Brain Injury: A Double-Edged Sword with Therapeutic Potentials.
Zhang H, Tian Y, Ma S, Ji Y, Wang Z, Xiao P, Xu Y. Zhang H, et al. Mol Neurobiol. 2024 Dec;61(12):10671-10683. doi: 10.1007/s12035-024-04230-4. Epub 2024 May 22. Mol Neurobiol. 2024. PMID: 38775879 Review. - Autophagy in the Immunosuppressive Perivascular Microenvironment of Glioblastoma.
Molina ML, García-Bernal D, Martinez S, Valdor R. Molina ML, et al. Cancers (Basel). 2019 Dec 31;12(1):102. doi: 10.3390/cancers12010102. Cancers (Basel). 2019. PMID: 31906065 Free PMC article. Review.
References
- Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–507. - PubMed
- Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. - PubMed
- Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. - PubMed
- Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32HL007910/HL/NHLBI NIH HHS/United States
- T32 HL007910/HL/NHLBI NIH HHS/United States
- R01AI1079065/AI/NIAID NIH HHS/United States
- T32 AI060519/AI/NIAID NIH HHS/United States
- T32AI060519/AI/NIAID NIH HHS/United States
- R01 AI079065/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous