The c-Jun N-terminal kinase (JNK) pathway is activated in human interstitial cystitis (IC) and rat protamine sulfate induced cystitis - PubMed (original) (raw)
The c-Jun N-terminal kinase (JNK) pathway is activated in human interstitial cystitis (IC) and rat protamine sulfate induced cystitis
Jiang Zhao et al. Sci Rep. 2016.
Abstract
The pathogenesis of bladder pain syndrome/interstitial cystitis (BPS/IC) is currently unclear. However, inflammation has been suggested to play an important role in BPS/IC. JNK downstream signaling plays an important role in numerous chronic inflammatory diseases. However, studies of the JNK pathway in BPS/IC are limited. In this study, we investigated the role of the JNK pathway in human BPS/IC and rat protamine sulfate (PS)-induced cystitis and examined the effect of the selective JNK inhibitor SP600125 on rat bladder cystitis. In our study, we demonstrated that the JNK signaling pathway was activated (the expression of JNK, c-Jun, p-JNK, p-c-Jun, IL-6 and TNF-α were significantly increasing in BPS/IC compared to the non-BPS/IC patients) and resulted in inflammation in human BPS/IC. Further animal models showed that the JNK pathway played an important role in the pathogenesis of cystitis. JNK inhibitors, SP600125, effectively inhibited the expression of p-JNK, p-c-Jun, IL-6 and TNF-α. The inhibition of these pathways had a protective effect on PS-induced rat cystitis by significantly decreasing histological score and mast cell count and improving bladder micturition function (micturition frequency significantly decreasing and bladder capacity significantly increasing). Therefore, JNK inhibition could be used as a potential treatment for BPS/IC.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
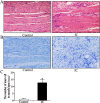
Figure 1. Histological evaluation in human BPS/IC.
(A,B) Representative HE and toluidine blue staining (x20) photomicrograph images of numerous inflammatory cells and mast cell infiltration into the bladder muscular layer, arrows demonstrate inflammatory cells and mast cell. (C) The chart indicates the number of mast cells in muscular layer in control (n = 7) and BPS/IC humans (n = 6). The data are expressed as the mean ± SD, *P < 0.05, BPS/IC vs. control.
Figure 2. Histological evaluation in rat PS-induced cystitis.
(A,B) Representative HE and toluidine blue staining (x20) photomicrograph images of pathologic changes and mast cell infiltration into the bladder muscular layer in PS-treated rats, arrows demonstrate mast cell.
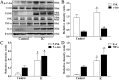
Figure 3. The expression of JNK, c-Jun, p-JNK, p-c-Jun, IL-6 and TNF-α changes in human BPS/IC using Western blot.
(A) Typical protein bands showing the increased expression of JNK, c-Jun, p-JNK, p-c-Jun, IL-6 and TNF-α in BPS/IC bladder. (B–D) The chart shows the expression of JNK, c-Jun, p-JNK, p-c-Jun, IL-6 and TNF-α in control (n = 8) and BPS/IC humans (n = 9). The data are expressed as the mean ± SD, *P < 0.05, BPS/IC vs. control.
Figure 4. The expression and location of p-JNK changes in human BPS/IC using immunohistochemistry.
(A) Representative immunohistochemistry (x20) shows p-JNK expression in the bladder muscles layers of mesenchymal cells (including inflammatory cells) and detrusor myocytes, arrows demonstrate the p-JNK expression. (B) The chart indicates the expression change of p-JNK in control (n = 7) and BPS/IC humans (n = 6). The data are expressed as the mean ± SD, *P < 0.05, BPS/IC vs. control.
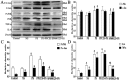
Figure 5. Evaluation of JNK, c-Jun, p-JNK, p-c-Jun, IL-6 and TNF-α changes in rat PS-induced cystitis using Western blot.
(A) Typical protein bands showing increased expression of JNK, c-Jun p-JNK, p-c-Jun, IL-6 and TNF-α in PS-treated rats. (B–D) The chart shows the expression of JNK, c-Jun, p-JNK, p-c-Jun, IL-6 and TNF-α in PS-treated rats (N = 8). The data are expressed as the mean ± SD, *P < 0.05, NS, PS, PPCES + PS and SP600125 + PS vs. control, ▲P < 0.05, SP600125 + PS vs. PS and PPCES + PS, #P < 0.05, SP600125 + PS vs. PS.
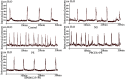
Figure 6. Representative cystometric traces of control, NS, PS, PPCES + PS and SP600125 + PS groups.
Similar articles
- Role of c-Jun N-terminal kinase (JNK) activation in micturition reflexes in cyclophosphamide (CYP)-induced cystitis in female rats.
Dugan C, Malley S, Arms L, May V, Vizzard MA. Dugan C, et al. J Mol Neurosci. 2014 Nov;54(3):360-9. doi: 10.1007/s12031-014-0308-5. Epub 2014 Apr 26. J Mol Neurosci. 2014. PMID: 24763745 Free PMC article. - Wnt/β-catenin signaling inhibits oxidative stress-induced ferroptosis to improve interstitial cystitis/bladder pain syndrome by reducing NF-κB.
Fang W, Song X, Li H, Meng F, Lv T, Huang J, Ji X, Lv J, Cai Z, Wang Z. Fang W, et al. Biochim Biophys Acta Mol Cell Res. 2024 Oct;1871(7):119766. doi: 10.1016/j.bbamcr.2024.119766. Epub 2024 May 31. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38823528 - Specific inhibition of ICAM-1 effectively reduces bladder inflammation in a rat model of severe non-bacterial cystitis.
Zhang X, He H, Lu G, Xu T, Qin L, Wang X, Jin X, Liu B, Zhao Z, Shen Z, Shao Y. Zhang X, et al. Sci Rep. 2016 Oct 26;6:35672. doi: 10.1038/srep35672. Sci Rep. 2016. PMID: 27782122 Free PMC article. - Pathology and terminology of interstitial cystitis/bladder pain syndrome: A review.
Akiyama Y, Homma Y, Maeda D. Akiyama Y, et al. Histol Histopathol. 2019 Jan;34(1):25-32. doi: 10.14670/HH-18-028. Epub 2018 Jul 17. Histol Histopathol. 2019. PMID: 30015351 Review. - Urinary IL-33 and galectin-3 increase in patients with interstitial cystitis/bladder pain syndrome (review).
Kochiashvili G, Kochiashvili D. Kochiashvili G, et al. Georgian Med News. 2014 Jul-Aug;(232-233):12-5. Georgian Med News. 2014. PMID: 25214264 Review.
Cited by
- Decreased autophagic activity of detrusor cells is involved in the inflammatory response of interstitial cystitis/bladder pain syndrome.
Zhao J, Lu Q, Yang Z, Sun B, Zhu J, Zhang H, Yang C, Yi S, Dong X. Zhao J, et al. Int Urogynecol J. 2023 Apr;34(4):843-851. doi: 10.1007/s00192-022-05224-3. Epub 2022 Jun 11. Int Urogynecol J. 2023. PMID: 35689690 - Network Pharmacology Approach to Investigate the Mechanism of Danggui-Shaoyao-San against Diabetic Kidney Disease.
Chen Y, Song X, Luo Y, Li G, Luo Y, Wang Z, He R, Lu J, Xiong G, Cheng H, Li H, Yang S. Chen Y, et al. Evid Based Complement Alternat Med. 2023 Jan 3;2023:9208017. doi: 10.1155/2023/9208017. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 36636607 Free PMC article. - Adenosine A2A Receptor Agonist Polydeoxyribonucleotide Alleviates Interstitial Cystitis-Induced Voiding Dysfunction by Suppressing Inflammation and Apoptosis in Rats.
Ko IG, Jin JJ, Hwang L, Kim SH, Kim CJ, Won KY, Na YG, Kim KH, Kim SJ. Ko IG, et al. J Inflamm Res. 2021 Feb 15;14:367-378. doi: 10.2147/JIR.S287346. eCollection 2021. J Inflamm Res. 2021. PMID: 33623409 Free PMC article. - Unraveling the complex roles of macrophages in obese adipose tissue: an overview.
Peng C, Chen J, Wu R, Jiang H, Li J. Peng C, et al. Front Med. 2024 Apr;18(2):205-236. doi: 10.1007/s11684-023-1033-7. Epub 2024 Jan 2. Front Med. 2024. PMID: 38165533 Review. - SNORC knockdown alleviates inflammation, autophagy defect and matrix degradation of chondrocytes in osteoarthritis development.
Tang Z, Feng H, Chen X, Shao S, Li C. Tang Z, et al. Mol Cell Biochem. 2024 Sep;479(9):2323-2335. doi: 10.1007/s11010-023-04842-9. Epub 2023 Sep 2. Mol Cell Biochem. 2024. PMID: 37659033
References
- Nickel J. C. et al. Psychosocial Phenotyping in Women With Interstitial Cystitis/Painful Bladder Syndrome: A Case Control Study. J Urol 183, 167–172 (2010). - PubMed
- Hanno P. et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn 29, 191–198 (2010). - PubMed
- Liu B. L. et al. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol Int 92, 202–208 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous