Mark4 promotes oxidative stress and inflammation via binding to PPARγ and activating NF-κB pathway in mice adipocytes - PubMed (original) (raw)
Mark4 promotes oxidative stress and inflammation via binding to PPARγ and activating NF-κB pathway in mice adipocytes
Zhenjiang Liu et al. Sci Rep. 2016.
Abstract
MAP/Microtubule affinity-regulating kinase 4 (Mark4) plays an important role in the regulation of microtubule organization, adipogenesis and apoptosis. However, the role of Mark4 plays in oxidative stress and inflammation are poorly understood. In this study, we found Mark4 was induced by high fat diet (HFD) while PPARγ was elevated significantly in mice adipocytes. Further analyses revealed Mark4 impaired mitochondrial oxidative respiration and increased reactive oxygen species (ROS) production. At same time, the activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) were greatly reduced. By treating cells with H2O2 and vitamin E (VE), Mark4 accentuated oxidative stress along with increased mRNA level of inflammatory factor interleukin-6 (IL-6) and decreased leptin mRNA. Furthermore, we found PPARγ bind to Mark4 promoter region and inhibited Mark4 expression. We showed PPARγ interacted with Mark4 and inhibited the stimulating effect of Mark4 on oxidative stress and inflammation. Finally, we demonstrated that the IKKα/NF-κB signal pathway was involved in Mark4 induced oxidative stress and inflammation, while PTDC, a special inhibitor of NF-κB signal pathway, reduced oxidative stress and inflammation. Thus, our study indicated that Mark4 was a potential drug target for treating metabolic diseases.
Figures
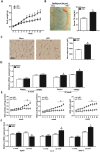
Figure 1. Mark4 expression is increased along with adipose oxidative stress and inflammation.
(A) Body weight of male mice fed HFD (n = 20 each). (B) EF pad representative picture of male mice fed HFD for 10 weeks. Mice serum TG content in both groups (HFD and chow diet, n = 16). (C) Representative hematoxylin and eosin (H&E) staining of EF pad tissue. And mean adipocyte area size (n = 16). (D) Relative mRNA level of Mark4 and PPARγ fed chow diet and HFD on 10th week (n = 16 each). (E) Activity of SOD, MDA, and ROS fed on chow diet and HFD for 10 weeks (n = 16 each). (F) Relative mRNA level of leptin, IL-6 and MCP-1 fed on chow diet and HFD on 10th week (n = 16 each). Values are means ± SD. vs. control group, *p < 0.05.
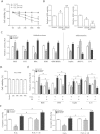
Figure 2. Mark4 blocks mitochondrial oxidative respiration in mice primary adipocyte.
(A) Mark4 transfection efficiency detection in Control group, HA-Mark4 group and sh-Mark4 group after 48 h transfection (n = 3). (B) Cell viability measurement in Control group, HA-Mark4 group and sh-Mark4 group after transfection with HA-Mark4 and sh-Mark4 for 12 h, 24 h and 48 h (n = 3). (C) mRNA level of Caspase3 after transfection with HA-Mark4 and sh-Mark4 for 48 h (n = 3). (D) Cyt C immunofluorescent staining after transfection with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte isolated from WAT of chow diet fed mice, and the detection of fluorescence intensity in control group, HA-Mark4 group and sh-Mark4 group. Scale bar: 100 μm (n = 3). (E) Immunoblots of Mark4 and Cyt C after transfection with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte (n = 3). (F) Immunofluorescent of JC-1 under a fluorescence microscope after transfected with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte isolated from WAT of chow diet fed mice, and the detection of fluorescence intensity in control group, HA-Mark4 group and sh-Mark4 group. Scale bar: 100 μm (n = 3). (G) Copy number of mtDNA after transfection with HA-Mark4 and sh-Mark4 in primary adipocyte for 48 h (n = 3). (H,I) After transfection with HA-Mark4 and sh-Mark4 for 48 h, the activity of mitochondrion complex I, III in primary adipocyte (n = 3). (J) The relative activity of ROS after transfection with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte (n = 3). (K) Relative mRNA levels of SOD, CAT, and GPx after transfection with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte. The level of total GAPDH was determined as loading control. Control: no transfection group, HA-Mark4 group: overexpression of Mark4 group, sh-Mark4 group: knock down of Mark4 group. Values are means ± SD. vs. control group, *p < 0.05.
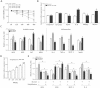
Figure 3. Mark4 promotes adipose oxidative stress.
(A) Primary adipocytes isolated from WAT of chow fed diet mice were cultured and incubated for 0 h, 12 h, 24 h, 36 h and 48 h in the presence of 100 nM or 200 nM H2O2. Cell viability was detected by CCK-8 (n = 3). (B) The relative ROS and SOD activity of the primary adipocytes incubated for 24 h in the presence of 100 nM H2O2 (n = 3). (C) Relative activity of ROS, CAT, GPx, SOD, GSH/GSSG, leptin, MCP-1 and IL-6 after transfection with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte. Before transfection primary adipocytes were pretreated with 100 nM H2O2 for 24 h (n = 3 each). (D) Isolated primary adipocytes were cultured and incubated for 0 h, 12 h, 24 h, 36 h and 48 h in the presence of 2 mM VE. Cell viability was detected by CCK8 (n = 3). (E) Relative activity of ROS, SOD, leptin and IL-6 after transfection with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte. Before transfection primary adipocytes were pretreated with 2 mM VE or 100 nM H2O2 for 24 h (n = 3). (F) After transfection with HA-Mark4 and sh-Mark4 for 48 h, the activity of mitochondrion complex I, III in primary adipocyte (n = 3). Before transfection primary adipocytes were pretreated with 2 mM VE or 100 nM H2O2 for 24 h (n = 3). Control: no transfection group, HA-Mark4 group: overexpression of Mark4 group, sh-Mark4 group: knock down of Mark4 group. Values are means ± SD. vs. control group, *p < 0.05, **p < 0.01.
Figure 4. Mark4 aggravates inflammation response in mice adipocytes.
(A) Primary adipocytes isolated from WAT of chow fed diet mice were cultured and incubated for 0 h, 12 h, 24 h, 36 h and 48 h in the presence of 5.6 nM, 15 nM and 30 nM glucose. Cell viability was detected by CCK-8 (n = 3). (B) Relative mRNA expression of Caspse3, Bcl-2/Bax, leptin, IL-6, PPARγ and Mark4 of the primary adipocytes incubated for 24 h in the presence of 15 nM glucose (n = 3). (C) Relative activity of ROS, SOD, CAT, MDA, IL-6, TNF-α, MCP-1 and leptin after transfection with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte. Before transfection, primary adipocytes were treated with 15 nM glucose for 24 h (n = 3). (D) Primary adipocytes were cultured and incubated for 0 h, 12 h, 24 h, 36 h and 48 h in the presence of 100 nM rosiglitazone for 24 h. Relative mRNA expression of PPARγ was detected (n = 3). (E) Relative activity of ROS, SOD, MCP-1 and IL-6 after transfection with HA-Mark4 and sh-Mark4 for 48 h in primary adipocyte. Before transfection primary adipocytes were pretreated with 15 nM glucose or 100 nM rosiglitazone for 24 h (n = 3). Control: no transfection group, HA-Mark4 group: overexpression of Mark4 group, sh-Mark4 group: knock down of Mark4 group. Values are means ± SD. vs. control group, *p < 0.05.
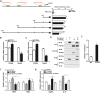
Figure 5. PPARγ inhibits adipose oxidative stress and inflammation by interacting directly with Mark4.
(A) Fragments of Mark4 promoter fused to a luciferase reporter gene were co-transfected into cells together with PGL3-basic (control) or pc-PPARγ (n = 3). Luciferase activity was corrected for Renilla luciferase activity and normalized to control activity (n = 3). (B) Relative mRNA expression of Mark4 and PPARγ of the primary adipocytes incubated for 24 h in the presence of 100 nM rosiglitazone (n = 3). (C) Relative mRNA expression of Mark4 and PPARγ of the primary adipocytes with pc-PPARγ transfected for 48 h (n = 3). (D) Mark4 interacted with PPARγ. Immunoprecipitation (IP) analysis was performed in HA-Mark4 and pc-PPARγ transfected cells (n = 3). (E) ChIP analysis of Mark4 and PPARγ in adipocytes (n = 3). (F) Relative activity of ROS and SOD after transfection with HA-Mark4 and pc-PPARγ for 48 h in primary adipocyte (n = 3). (G) Relative mRNA of leptin and IL-6 after transfection with HA-Mark4 and pc-PPARγ for 48 h in primary adipocyte (n = 3). Control: transfection of pcDNA3.1-vector group, HA-Mark4 group: overexpression of Mark4 group, pc-PPARγ: overexpression of PPARγ group. Values are means ± SD. vs. control group, *p < 0.05.
Figure 6. IKKα/NF-κB signal is essential for Mark4 activated adipose oxidative stress and inflammation.
(A) Representative immunoblots and densitometric quantification for Mark4, p-IKKα (T23) and p-NF-κB (S337) after transfection with HA-Mark4, sh-Mark4 and PTDC for 48 h in primary adipocyte (n = 3). (B) Representative immunoblots and densitometric quantification for IL-6, MCP-1, SOD, Cyt C and PGC1-α after transfection with HA-Mark4, sh-Mark4 and PTDC for 48 h in primary adipocyte (n = 3). The level of total GAPDH was determined as loading control. Control: no transfection group, HA-Mark4 group: overexpression of Mark4 group, sh-Mark4 group: knock down of Mark4 group. Values are means ± SD. vs. control group, *p < 0.05.
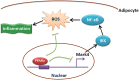
Figure 7. Summary of Mark4 in the regulation of oxidative stress and inflammation via IKKα/NF-κB signaling pathway in murine adipocytes.
And PPARγ binding to Mark4 as a transcriptional suppressor in this regulation progress.
Similar articles
- Mark4 promotes adipogenesis and triggers apoptosis in 3T3-L1 adipocytes by activating JNK1 and inhibiting p38MAPK pathways.
Feng M, Tian L, Gan L, Liu Z, Sun C. Feng M, et al. Biol Cell. 2014 Sep;106(9):294-307. doi: 10.1111/boc.201400004. Epub 2014 Aug 6. Biol Cell. 2014. PMID: 24989893 - Rhizoma Dioscoreae Nipponicae polysaccharides protect HUVECs from H2O2-induced injury by regulating PPARγ factor and the NADPH oxidase/ROS-NF-κB signal pathway.
Jin Y, Liu K, Peng J, Wang C, Kang L, Chang N, Sun H. Jin Y, et al. Toxicol Lett. 2015 Jan 5;232(1):149-58. doi: 10.1016/j.toxlet.2014.10.006. Epub 2014 Oct 8. Toxicol Lett. 2015. PMID: 25305479 - Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Kauppinen A, et al. Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review. - Oxidative stress and redox regulation of lung inflammation in COPD.
Rahman I, Adcock IM. Rahman I, et al. Eur Respir J. 2006 Jul;28(1):219-42. doi: 10.1183/09031936.06.00053805. Eur Respir J. 2006. PMID: 16816350 Review.
Cited by
- Crosstalk Between Antioxidants and Adipogenesis: Mechanistic Pathways and Their Roles in Metabolic Health.
Fu M, Yoon KS, Ha J, Kang I, Choe W. Fu M, et al. Antioxidants (Basel). 2025 Feb 10;14(2):203. doi: 10.3390/antiox14020203. Antioxidants (Basel). 2025. PMID: 40002389 Free PMC article. Review. - Suberoylanilide Hydroxamic Acid Attenuates Interleukin-1β-Induced Interleukin-6 Upregulation by Inhibiting the Microtubule Affinity-Regulating Kinase 4/Nuclear Factor-κB Pathway in Synovium-Derived Mesenchymal Stem Cells from the Temporomandibular Joint.
Sun J, Liao W, Su K, Jia J, Qin L, Liu W, He Y, Zhang H, Ou F, Zhang Z, Sun Y. Sun J, et al. Inflammation. 2020 Aug;43(4):1246-1258. doi: 10.1007/s10753-020-01204-1. Inflammation. 2020. PMID: 32279160 - Regulation of Cell Polarity by PAR-1/MARK Kinase.
Wu Y, Griffin EE. Wu Y, et al. Curr Top Dev Biol. 2017;123:365-397. doi: 10.1016/bs.ctdb.2016.11.001. Epub 2016 Dec 5. Curr Top Dev Biol. 2017. PMID: 28236972 Free PMC article. Review. - Increased Myocardial MARK4 Expression in Patients with Heart Failure and Sleep-Disordered Breathing.
Seydel B, Hegner P, Lauerer AM, Schildt S, Bayram F, Tafelmeier M, Wermers D, Rupprecht L, Schmid C, Wagner S, Maier LS, Arzt M, Lebek S. Seydel B, et al. Int J Mol Sci. 2025 Apr 11;26(8):3614. doi: 10.3390/ijms26083614. Int J Mol Sci. 2025. PMID: 40332117 Free PMC article. - Anti-Oxidative Effects of Melatonin Receptor Agonist and Omega-3 Polyunsaturated Fatty Acids in Neuronal SH-SY5Y Cells: Deciphering Synergic Effects on Anti-Depressant Mechanisms.
Satyanarayanan SK, Shih YH, Chien YC, Huang SY, Gałecki P, Kasper S, Chang JP, Su KP. Satyanarayanan SK, et al. Mol Neurobiol. 2018 Sep;55(9):7271-7284. doi: 10.1007/s12035-018-0899-x. Epub 2018 Feb 3. Mol Neurobiol. 2018. PMID: 29397559
References
- Hurov J. & Piwnica-Worms H. The Par-1/MARK Family of Protein Kinases. Cell Cycle 6, 1966–1969 (2007). - PubMed
- Naz F., Anjum F., Islam A., Ahmad F. & Hassan M. I. Microtubule Affinity-Regulating Kinase 4: Structure, Function, and Regulation. Cell Biochem Biophys 67, 485–499 (2013). - PubMed
- Rovina D. et al. Microtubule-associated protein/microtubule affinity-regulating kinase 4 (MARK4) plays a role in cell cycle progression and cytoskeletal dynamics. Eur J Cell Biol. 93, 355–365 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous