Interleukin-6 is a potential therapeutic target in interleukin-6 dependent, estrogen receptor-α-positive breast cancer - PubMed (original) (raw)
Interleukin-6 is a potential therapeutic target in interleukin-6 dependent, estrogen receptor-α-positive breast cancer
Tineke Casneuf et al. Breast Cancer (Dove Med Press). 2016.
Abstract
Introduction: Interleukin-6 (IL-6) is an important growth factor for estrogen receptor-α (ERα)-positive breast cancer, and elevated serum IL-6 is associated with poor prognosis.
Methods: The role of the phosphorylated signal transducer and activator of transcription 3 pathway was investigated in ERα-positive breast cancer. A panel of cell lines was treated with exogenous IL-6. An IL-6 specific gene signature was generated by profiling ten ERα-positive breast cancer cell lines alone or following treatment with 10 ng/mL recombinant IL-6 or human marrow stromal cell-conditioned media, with or without siltuximab (a neutralizing anti-IL-6 antibody) and grown in three-dimensional tumor microenvironment-aligned cultures for 4 days, 5 days, or 6 days. The established IL-6 signature was validated against 36 human ERα-positive breast tumor samples with matched serum. A comparative MCF-7 xenograft murine model was utilized to determine the role of IL-6 in estrogen-supplemented ERα-positive breast cancer to assess the efficacy of anti-IL-6 therapy in vivo.
Results: In eight of nine ERα-positive breast cancer cell lines, recombinant IL-6 increased phosphorylation of tyrosine 705 of STAT3. Differential gene expression analysis identified 17 genes that could be used to determine IL-6 pathway activation by combining their expression intensity into a pathway activation score. The gene signature included a variety of genes involved in immune cell function and migration, cell growth and apoptosis, and the tumor microenvironment. Validation of the IL-6 gene signature in 36 matched human serum and ERα-positive breast tumor samples showed that patients with a high IL-6 pathway activation score were also enriched for elevated serum IL-6 (≥10 pg/mL). When human IL-6 was provided in vivo, MCF-7 cells engrafted without the need for estrogen supplementation, and addition of estrogen to IL-6 did not further enhance engraftment. Subsequently, we prophylactically treated mice at MCF-7 engraftment with siltuximab, fulvestrant, or combination therapy. Siltuximab alone was able to blunt MCF-7 engraftment. Similarly, siltuximab alone induced regressions in 90% (9/10) of tumors, which were established in the presence which were established in the presence of hMSC expressing human IL-6 and estrogen.
Conclusion: Given the established role for IL-6 in ERα-positive breast cancer, these data demonstrate the potential for anti-IL-6 therapeutics in breast cancer.
Keywords: breast cancer; estrogen receptor; gene signature; paracrine IL-6; siltuximab.
Figures
Figure 1
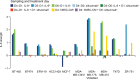
Effect of recombinant IL-6 on STAT3-Tyr705 phosphorylation in ERα-positive and ERα-negative cell lines. Notes: Cell lines were treated with IL-6 and analyzed at baseline and 24 hours posttreatment. Each blot represents independent experiments and bands were digitally excised without modification and rearranged for presentation. Abbreviations: IL-6, interleukin-6; pSTAT3, phosphorylated signal transducer and activator of transcription 3; ER, estrogen receptor.
Figure 2
Effect of recombinant IL-6 on Akt, MEK 1/2, and ERK 1/2 phosphorylation in ERα-positive and ERα-negative cell lines. Notes: Cell lines were treated with IL-6 and analyzed at baseline and 24 hours posttreatment. Each blot represents independent experiments and bands were digitally excised without modification and rearranged for presentation. Abbreviations: IL-6, interleukin-6; Akt, serine-threonine kinase; MEK, MAPK/ERK kinase; ERK, extracellular signal regulated kinase; ER, estrogen receptor.
Figure 3
Strength of the IL-6 signature in ERα-positive breast cancer cell lines at different time points and under different treatment conditions. Notes: Bar graphs represent each sampling time point (days 4–6) following administration of additional treatment(s) at either day 0 or day 1. aD4–D1: IL-6: cell lines treated with IL-6 on day 1, harvested on day 4. bD5–D1: IL-6: cell lines treated with IL-6 on day 1, harvested on day 5. cD6–D1: IL-6: cell lines treated with IL-6 on day 1, harvested on day 6. dD4–D0: siltuximab: cell lines treated with siltuximab on day 0, harvested on day 4. eD4–D0: IL-6 + D1: siltuximab: cell lines treated with IL-6 on day 0, with siltuximab on day 1, harvested on day 4. fD4–D1: IL6 + D1: siltuximab: cell lines treated with IL-6 and siltuximab on day 1, harvested on day 4. gD4: hMSC-CM: hMSC-CM harvested on day 4. hD4: hMSC-CM + D0: siltuximab: hMSC-CM grown in presence of siltuximab as of day 0, harvested on day 4. Abbreviations: D, day; IL-6, interleukin-6; ER, estrogen receptor; hMSC-CM, human marrow stromal cell-conditioned media.
Figure 4
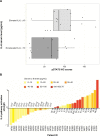
The relationship between elevated serum IL-6 and increased intratumoral phosphorylated STAT3-Y705 in human breast cancer samples. Notes: (A) Elevated IL-6 versus nonelevated IL-6 serum levels were analyzed using the Wilcoxon rank sum test. pSTAT3 was analyzed using immunohistochemistry (IHC); serum IL-6 was analyzed using a panoptic IL-6 meso scale detection (MSD) assay, _P_=0.04. Seven of 36 breast cancer samples were not profiled for pSTAT3 immunohistochemistry; and therefore, only 29 samples are represented in the figure. (B) Waterfall plot of IL-6 pathway activation score versus serum IL-6 concentration in human breast cancer samples. Abbreviations: IL-6, interleukin-6; pSTAT3, phosphorylated signal transducer and activator of transcription 3.
Figure 5
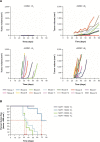
Summary of findings from MCF-7 murine tumor xenograft model. Notes: (A) 5×106 MCF-7 cells were coinjected into the murine mammary fat pad with either estrogen pellets (E2), human mesenchymal stromal cells (hMSCs, 0.5×106), or both. Top left, neither hMSC nor E2; top right, E2 only; bottom left, hMSC only; bottom right, hMSC + E2. (B) Percentages of animals demonstrating tumor volume ≥500 mm3.
Figure 6
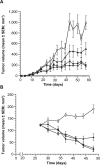
Changes in tumor volume in the MCF-7 murine tumor xenograft model over a 6-week period. Notes: (A) Prophylactic treatment with 1) control (vehicle, □); 2) siltuximab 20 mg/kg bodyweight twice weekly (▲); 3) fulvestrant 200 mg/kg bodyweight once weekly (▼); or 4) siltuximab + fulvestrant (♦). Each treatment group contained ten mice. (B) Treatment of established tumors (volume 100–150 mm3) with 1) control (vehicle, □); 2) siltuximab 20 mg/kg bodyweight twice weekly (▲); 3) fulvestrant 200 mg/kg bodyweight once weekly (▼); or 4) siltuximab + fulvestrant (♦). Each treatment group contained ten mice. Abbreviation: SEM, standard error of the mean.
Figure 7
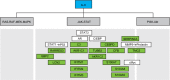
Functional interpretation of the IL-6 gene signature and its relationship to the three main downstream pathways of IL-6. Notes: The 17 genes are part of the Jak-STAT IL-6 downstream pathway. Members of the gene signature are represented by green boxes, and upstream pathway members are represented by white boxes. Abbreviations: IL-6, interleukin-6; MEK, MAPK/ERK kinase; MAPK, mitogen-activated protein kinase; PI3K, phosphatidyl-inositol-3-kinase; Akt, serine-threonine kinase; STAT3, signal transducer and activator of transcription 3; CEBPD, CCAAT/enhancer binding protein; MMP9, matrix metalloproteinase 9; TUBB3, β-tubulin isotype III; CFB, complement factor B; TMC-5, transmembrane channel-like protein 5; GBP2, guanylate binding protein 2; AKR, aldoketone reductase; LCN2, lipocalin 2; IFITM, interferon-inducible transmembrane protein.
Similar articles
- Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer.
Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Sasser AK, et al. FASEB J. 2007 Nov;21(13):3763-70. doi: 10.1096/fj.07-8832com. Epub 2007 Jun 22. FASEB J. 2007. PMID: 17586727 - Reawakening of dormant estrogen-dependent human breast cancer cells by bone marrow stroma secretory senescence.
Tivari S, Lu H, Dasgupta T, De Lorenzo MS, Wieder R. Tivari S, et al. Cell Commun Signal. 2018 Aug 17;16(1):48. doi: 10.1186/s12964-018-0259-5. Cell Commun Signal. 2018. PMID: 30119678 Free PMC article. - Interleukin-10: A double-edged sword in breast cancer.
Chang CM, Lam HYP, Hsu HJ, Jiang SJ. Chang CM, et al. Tzu Chi Med J. 2021 Feb 24;33(3):203-211. doi: 10.4103/tcmj.tcmj_162_20. eCollection 2021 Jul-Sep. Tzu Chi Med J. 2021. PMID: 34386356 Free PMC article. Review. - The role of IL-35 and IL-37 in breast cancer - potential therapeutic targets for precision medicine.
Ma Y, Su H, Wang X, Niu X, Che Y, Hambly BD, Bao S, Wang X. Ma Y, et al. Front Oncol. 2022 Nov 22;12:1051282. doi: 10.3389/fonc.2022.1051282. eCollection 2022. Front Oncol. 2022. PMID: 36483045 Free PMC article. Review.
Cited by
- Dysregulated tumor-associated macrophages in carcinogenesis, progression and targeted therapy of gynecological and breast cancers.
Xu T, Yu S, Zhang J, Wu S. Xu T, et al. J Hematol Oncol. 2021 Oct 30;14(1):181. doi: 10.1186/s13045-021-01198-9. J Hematol Oncol. 2021. PMID: 34717710 Free PMC article. Review. - Altered Toll-like receptor expression and function in HPV-associated oropharyngeal carcinoma.
Tobouti PL, Bolt R, Radhakrishnan R, de Sousa SCOM, Hunter KD. Tobouti PL, et al. Oncotarget. 2017 Jul 4;9(1):236-248. doi: 10.18632/oncotarget.18959. eCollection 2018 Jan 2. Oncotarget. 2017. PMID: 29416610 Free PMC article. - Small GTPase RBJ promotes cancer progression by mobilizing MDSCs via IL-6.
Liu Q, Zhu H, Zhang C, Chen T, Cao X. Liu Q, et al. Oncoimmunology. 2016 Dec 23;6(1):e1245265. doi: 10.1080/2162402X.2016.1245265. eCollection 2017. Oncoimmunology. 2016. PMID: 28197363 Free PMC article. - IL-6: The Link Between Inflammation, Immunity and Breast Cancer.
Chen J, Wei Y, Yang W, Huang Q, Chen Y, Zeng K, Chen J. Chen J, et al. Front Oncol. 2022 Jul 18;12:903800. doi: 10.3389/fonc.2022.903800. eCollection 2022. Front Oncol. 2022. PMID: 35924148 Free PMC article. Review. - Novel mechanism for OSM-promoted extracellular matrix remodeling in breast cancer: LOXL2 upregulation and subsequent ECM alignment.
Dinca SC, Greiner D, Weidenfeld K, Bond L, Barkan D, Jorcyk CL. Dinca SC, et al. Breast Cancer Res. 2021 May 19;23(1):56. doi: 10.1186/s13058-021-01430-x. Breast Cancer Res. 2021. PMID: 34011405 Free PMC article.
References
- Ji Q, Aoyama C, Nien YD, et al. Selective loss of AKR1C1 and AKR1C2 in breast cancer and their potential effect on progesterone signaling. Cancer Res. 2004;64(20):7610–7617. - PubMed
- Gery S, Tanosaki S, Hofmann WK, Koppel A, Koeffler HP. C/EBPdelta expression in a BCR-ABL-positive cell line induces growth arrest and myeloid differentiation. Oncogene. 2005;24(9):1589–1597. - PubMed
- Naderi A, Teschendorff AE, Barbosa-Morais NL, et al. A gene-expression signature to predict survival in breast cancer across independent data sets. Oncogene. 2007;26(10):1507–1516. - PubMed
- Godoy P, Cadenas C, Hellwig B, et al. Interferon-inducible guanylate binding protein (GBP2) is associated with better prognosis in breast cancer and indicates an efficient T cell response. Breast Cancer. 2014;21(4):491–499. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous