Ezrin Enhances EGFR Signaling and Modulates Erlotinib Sensitivity in Non-Small Cell Lung Cancer Cells - PubMed (original) (raw)
Ezrin Enhances EGFR Signaling and Modulates Erlotinib Sensitivity in Non-Small Cell Lung Cancer Cells
Yasemin Saygideğer-Kont et al. Neoplasia. 2016 Feb.
Abstract
Ezrin is a scaffolding protein that is involved in oncogenesis by linking cytoskeletal and membrane proteins. Ezrin interacts with epidermal growth factor receptor (EGFR) in the cell membrane, but little is known about the effects of this interaction on EGFR signaling pathway. In this study, we established the biological and functional significance of ezrin-EGFR interaction in non-small cell lung cancer (NSCLC) cells. Endogenous ezrin and EGRF interaction was confirmed by co-immunoprecipitation and immunofluorescent staining. When expression of ezrin was inhibited, EGFR activity and phosphorylation levels of downstream signaling pathway proteins ERK and STAT3 were decreased. Cell fractionation experiments revealed that nuclear EGFR was significantly diminished in ezrin-knockdown cells. Consequently, mRNA levels of EGFR target genes AURKA, COX-2, cyclin D1, and iNOS were decreased in ezrin-depleted cells. A small molecule inhibitor of ezrin, NSC305787, reduced EGF-induced phosphorylation of EGFR and downstream target proteins, EGFR nuclear translocation, and mRNA levels of nuclear EGFR target genes similar to ezrin suppression. NSC305787 showed synergism with erlotinib in wild-type EGFR-expressing NSCLC cells, whereas no synergy was observed in EGFR-null cells. Phosphorylation of ezrin on Y146 was found as an enhancer of ezrin-EGFR interaction and required for increased proliferation, colony formation, and drug resistance to erlotinib. These findings suggest that ezrin-EGFR interaction augments oncogenic functions of EGFR and that targeting ezrin may provide a potential novel approach to overcome erlotinib resistance in NSCLC cells.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Supplementary Figure S1
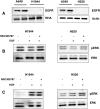
Ezrin inhibitor NSC305787 shows no effect on ERK phosphorylation in two EGFR-null cell lines. (A) Western blot analysis of EGFR levels in H1944 and H520 cells. A549 cell line was used as a positive control. (B) Cells were serum starved for 24 hours and treated with 5 μM NSC305787 or DMSO for 30 minutes before EGF treatment for 5 minutes. (C) Cells were serum starved for 24 hours and treated with 5 μM NSC305787 or DMSO for 30 minutes before HGF treatment for 5 minutes.
Supplementary Figure S2
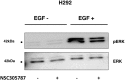
_N_SC305787 did not affect pERK levels in EGF − H292 cells. H292 cells starved overnight and treated with NSC305787 or DMSO in EGF − and EGF + conditions. There was no effect on pERK levels in EGF − H292 cells.
Supplementary Figure S3
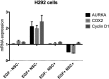
NSC305787 did not affect mRNA levels of nEGFR target genes in EGF − H292 cells. H292 cells starved overnight and treated with NSC305787 or DMSO in EGF − and EGF + conditions. There was no effect on mRNA levels in EGF − H292 cells.
Supplementary Figure S4
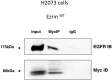
Ezrin wt did interact with EGFR. Ezrin-EGFR interaction in H2073 cells transfected with cDNAs coding for ezrin wt was analyzed by immunoprecipitation using anti-myc antibody followed by Western blotting.
Supplementary Figure S5
Ezrin Y353E or Y353F mutants do not interact with EGFR. Ezrin-EGFR interaction in H2073 cells transfected with cDNAs coding for ezrin Y353E and Y353F mutants was analyzed by immunoprecipitation using anti-myc antibody followed by Western blotting.
Supplementary Figure S6
Ezrin Y146E mutant does not affect proliferation of H520 cells. EGFR-null H520 cells were transfected with cDNAs coding for Ezrin wt and Y146E and Y146F mutant forms, and cell proliferation was assessed by MTT assay after 72 hours. Western blot analysis 25 shows levels of ezrin expression after transfection of H520 cells with corresponding expression constructs.
Figure 1
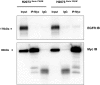
Ezrin and EGFR interacts in NSCLC cell lines. (A) H292 cells were serum starved overnight and pretreated with 5 μM ezrin inhibitor NSC305787 or DMSO for 2 hours followed by 100-ng/ml EGF treatment for 5 minutes. Cell lysates were immunoprecipitated using an antiezrin antibody or control mouse total IgG. Precipitates were evaluated by Western blotting using anti-EGFR (upper panel) and antiezrin (lower panel) antibodies. EGFR interaction with ezrin was enhanced with EGF stimulation, and NSC305787 inhibited this interaction in both the presence and absence of the ligand. (B) Immunofluorescence staining of EGFR (red), ezrin (green), and DAPI (blue) in DMSO-treated (upper images) and NSC305787-treated (bottom images) A549 cells was analyzed by fluorescence microscopy. Ezrin and EGFR colocalization (yellow) in the membrane (arrow) and perinuclear area (arrow head) is indicated. NSC305787 treatment inhibited the colocalization of ezrin and EGFR.
Figure 2
Reduced ezrin expression inhibits EGFR and phosphorylation of downstream target proteins in NSCLC cells. (A) Expression of ezrin was inhibited using siRNA oligos in A549 and H292 cells. Serum-starved cells were stimulated with 100 ng/ml of EGF for 5 minutes. EGFR activity was determined by immunoblot using phosphospecific antibodies for Y1068 and Y845. (B) Phosphorylation of EGFR downstream pathway proteins ERK and STAT3 was determined in cell lysates from panel A. Similar to EGFR, phospho-STAT3 and phospho-ERK levels were decreased in ezrin-depleted cells. (C) Cells were serum starved for 24 hours and treated with 5 μM NSC305787 or DMSO for 30 minutes before 5 minutes of EGF treatment. Phospho-STAT3 and phospho-ERK protein levels were decreased in NSC305787-treated cells.
Figure 3
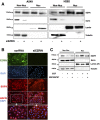
Inhibition of ezrin expression causes decreased levels of EGFR in nucleus in NSCLC cells. (A) Ezrin expression was inhibited using siRNA oligos in A549 and H292 cells, and whole cell lysates were subjected to cell fractionation and immunoblotting with antiezrin and anti-EGFR antibodies. Lamin A/C and tubulin antibodies were used as control for nuclear and non-nuclear fractions, respectively. (B) Immunofluorescence staining of EGFR, ezrin, and DAPI in control siRNA–treated (left panel) and ezrin siRNA–treated (right panel) A549 cells. Nuclear and perinuclear EGFR localization was decreased in ezrin-depleted cells. (C) Cells were serum starved for 24 hours and treated with 5 μM NSC305787 or DMSO for 2 hours before EGF stimulation for 30 minutes. NSC305787 decreased EGF-induced nuclear translocation of EGFR in H292 cells.
Figure 4
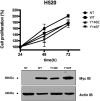
Suppression of ezrin expression and its pharmacological inhibition by NSC305787 causes decreased mRNA levels of nEGFR target genes in EGFR wt NSCLC cells_._ (A) mRNA expression levels of indicated genes were analyzed by quantitative PCR in ezrin-depleted A549 cells. CT values were normalized to 18S rRNA, and fold differences were calculated using the comparative CT method. (B) NSC305787 treatment did not decrease mRNA levels of nEGFR target genes in EGFR-null H520 cells, whereas AURKA and COX-2 levels were decreased in wt EGFR H292 cells. (C) Ezrin and EGFR protein expression levels were determined by immunoblotting after transfection of A549, H292, and H520 cells with siRNA oligos targeting ezrin. (D) Proliferation of A549, H292, and H520 cells after transfection with siRNA oligos targeting ezrin was determined by MTT assay. Ezrin knockdown significantly decreased proliferation of wt EGFR-expressing cells, A549 and H292, and did not affect EGFR-null cells, H520. (*P < .01, unpaired t test with Welch’s correction).
Figure 5
Ezrin knockdown sensitizes NSCLC cells to erlotinib treatment. MTT test was used to evaluate the cell viability. (A) Nonlinear curve fit and (B) IC50 values of control siRNA (siCTRL)– and siEZR-treated A549, H292, and H520 cells are given. Reduced ezrin expression caused a decreased IC50 value for erlotinib in A549 and H292 cells compared with H520 cells. (C) Ezrin inhibitor NSC305787 shows synergism with erlotinib in A549 cells. No synergy was observed in H520 cells treated with erlotinib-NSC305787 combination (D) and in A549 cells treated with etoposide-NSC305787 combination (E).
Figure 6
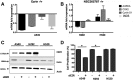
Y146 phosphorylation of ezrin affects its interaction with EGFR. (A) The 100-ng/ml EGF treatment induces tyrosine phosphorylation of ezrin on Y146 and Y353 residues in A549 cells. (B) Protein expression levels of myc-tagged Ezrinwt, Y146E, and Y146F were determined by immunoblotting following transfection of H2073 cells with corresponding expression constructs. (C) Ezrin-EGFR interaction in H2073 cells transfected with cDNAs coding for ezrin Y146E and Y146F mutants was determined by immunoprecipitation using anti-myc antibody followed by Western blotting. (D) Ectopic expression of ezrinwt and ezrin Y146E mutant sensitizes cells to erlotinib in H2073 cells. IC50 values are given in the table, and cell viability curves are shown in the graph. (E) The number of colonies in H2073 cells transfected with cDNAs coding for ezrin Y145E and Y145F mutants was determined by soft agar colony formation assay (*P < .01, unpaired t test with Welch’s correction). (F) Ectopic overexpression of ezrinwt and ezrin Y146E mutant caused an increased proliferation of H2073 cells compared with ezrin Y146F mutant.
Similar articles
- Exploring the mechanism of non-small-cell lung cancer cell lines resistant to epidermal growth factor receptor tyrosine kinase inhibitor.
Yu Y, Luo Y, Zheng Y, Zheng X, Li W, Yang L, Jiang J. Yu Y, et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):121-5. doi: 10.4103/0973-1482.151425. J Cancer Res Ther. 2016. PMID: 27072223 - Focal Adhesion Kinase Inhibitors in Combination with Erlotinib Demonstrate Enhanced Anti-Tumor Activity in Non-Small Cell Lung Cancer.
Howe GA, Xiao B, Zhao H, Al-Zahrani KN, Hasim MS, Villeneuve J, Sekhon HS, Goss GD, Sabourin LA, Dimitroulakos J, Addison CL. Howe GA, et al. PLoS One. 2016 Mar 10;11(3):e0150567. doi: 10.1371/journal.pone.0150567. eCollection 2016. PLoS One. 2016. PMID: 26962872 Free PMC article. - Tyr1068-phosphorylated epidermal growth factor receptor (EGFR) predicts cancer stem cell targeting by erlotinib in preclinical models of wild-type EGFR lung cancer.
Sette G, Salvati V, Mottolese M, Visca P, Gallo E, Fecchi K, Pilozzi E, Duranti E, Policicchio E, Tartaglia M, Milella M, De Maria R, Eramo A. Sette G, et al. Cell Death Dis. 2015 Aug 6;6(8):e1850. doi: 10.1038/cddis.2015.217. Cell Death Dis. 2015. PMID: 26247735 Free PMC article. - Acquired resistance of non-small cell lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors.
Nurwidya F, Takahashi F, Murakami A, Kobayashi I, Kato M, Shukuya T, Tajima K, Shimada N, Takahashi K. Nurwidya F, et al. Respir Investig. 2014 Mar;52(2):82-91. doi: 10.1016/j.resinv.2013.07.007. Epub 2013 Aug 30. Respir Investig. 2014. PMID: 24636263 Review. - Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer.
Gadgeel SM, Wozniak A. Gadgeel SM, et al. Clin Lung Cancer. 2013 Jul;14(4):322-32. doi: 10.1016/j.cllc.2012.12.001. Epub 2013 Jan 16. Clin Lung Cancer. 2013. PMID: 23332287 Review.
Cited by
- Nuclear epidermal growth factor receptor (nEGFR) in clinical treatment.
Zhu J, Wu Z, Shan G, Huang Y, Liang J, Zhan C. Zhu J, et al. Heliyon. 2024 Nov 5;10(21):e40150. doi: 10.1016/j.heliyon.2024.e40150. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39568844 Free PMC article. Review. - Ezrin's role in gastric cancer progression: Implications for immune microenvironment modulation and therapeutic potential.
Zhu Y, Zhang X, Chen Y, Liu Q, Yang J, Fan X, Song H, Cheng Z, Liu S. Zhu Y, et al. Heliyon. 2024 Feb 28;10(5):e27155. doi: 10.1016/j.heliyon.2024.e27155. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38449647 Free PMC article. - Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis.
Buenaventura RGM, Merlino G, Yu Y. Buenaventura RGM, et al. Cells. 2023 Jun 14;12(12):1620. doi: 10.3390/cells12121620. Cells. 2023. PMID: 37371090 Free PMC article. Review. - Targeting the Ezrin Adaptor Protein Sensitizes Metastatic Breast Cancer Cells to Chemotherapy and Reduces Neoadjuvant Therapy-induced Metastasis.
Hoskin V, Ghaffari A, Laight BJ, SenGupta S, Madarnas Y, Nicol CJB, Elliott BE, Varma S, Greer PA. Hoskin V, et al. Cancer Res Commun. 2022 Jun 17;2(6):456-470. doi: 10.1158/2767-9764.CRC-21-0117. eCollection 2022 Jun. Cancer Res Commun. 2022. PMID: 36923551 Free PMC article. - HAS2-Ezrin-ER axis plays a role in acquired antiestrogen resistance of ER-positive breast cancer.
Sun X, Tang F, Guo Q, Liu Y, He Y, Du Y, Gao F, Zhang G, Yang C. Sun X, et al. Front Pharmacol. 2022 Oct 31;13:1031487. doi: 10.3389/fphar.2022.1031487. eCollection 2022. Front Pharmacol. 2022. PMID: 36386154 Free PMC article.
References
- Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102(1):37–46. - PubMed
- Merrick DT, Kittelson J, Winterhalder R, Kotantoulas G, Ingeberg S, Keith RL, Kennedy TC, Miller YE, Franklin WA, Hirsch FR. Analysis of c-ErbB1/epidermal growth factor receptor and c-ErbB2/HER-2 expression in bronchial dysplasia: evaluation of potential targets for chemoprevention of lung cancer. Clin Cancer Res. 2006;12(7 Pt 1):2281–2288. - PubMed
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12(18):5268–5272. - PubMed
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2–16. - PubMed
- Wiley HS. Trafficking of the ErbB receptors and its influence on signaling. Exp Cell Res. 2003;284(1):78–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous