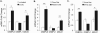miRNA Targeting of Oxysterol-Binding Protein-Like 6 Regulates Cholesterol Trafficking and Efflux - PubMed (original) (raw)
. 2016 May;36(5):942-951.
doi: 10.1161/ATVBAHA.116.307282. Epub 2016 Mar 3.
Elizabeth J Hennessy # 1, Coen van Solingen 1, Graeme J Koelwyn 1, Maryem A Hussein 2, Bhama Ramkhelawon 1, Katey J Rayner 3, Ryan E Temel 4, Ljubica Perisic 5, Ulf Hedin 5, Lars Maegdefessel 6, Michael J Garabedian 2, Lesca M Holdt 7, Daniel Teupser 7, Kathryn J Moore 1
Affiliations
- PMID: 26941018
- PMCID: PMC4850101
- DOI: 10.1161/ATVBAHA.116.307282
miRNA Targeting of Oxysterol-Binding Protein-Like 6 Regulates Cholesterol Trafficking and Efflux
Mireille Ouimet et al. Arterioscler Thromb Vasc Biol. 2016 May.
Abstract
Objective: Cholesterol homeostasis is fundamental to human health and is, thus, tightly regulated. MicroRNAs exert potent effects on biological pathways, including cholesterol metabolism, by repressing genes with related functions. We reasoned that this mode of pathway regulation could be exploited to identify novel genes involved in cholesterol homeostasis.
Approach and results: Here, we identify oxysterol-binding protein-like 6 (OSBPL6) as a novel target of 2 miRNA hubs regulating cholesterol homeostasis: miR-33 and miR-27b. Characterization of OSBPL6 revealed that it is transcriptionally regulated in macrophages and hepatocytes by liver X receptor and in response to cholesterol loading and in mice and nonhuman primates by Western diet feeding. OSBPL6 encodes the OSBPL-related protein 6 (ORP6), which contains dual membrane- and endoplasmic reticulum-targeting motifs. Subcellular localization studies showed that ORP6 is associated with the endolysosomal network and endoplasmic reticulum, suggesting a role for ORP6 in cholesterol trafficking between these compartments. Accordingly, knockdown of OSBPL6 results in aberrant clustering of endosomes and promotes the accumulation of free cholesterol in these structures, resulting in reduced cholesterol esterification at the endoplasmic reticulum. Conversely, ORP6 overexpression enhances cholesterol trafficking and efflux in macrophages and hepatocytes. Moreover, we show that hepatic expression of OSBPL6 is positively correlated with plasma levels of high-density lipoprotein cholesterol in a cohort of 200 healthy individuals, whereas its expression is reduced in human atherosclerotic plaques.
Conclusions: These studies identify ORP6 as a novel regulator of cholesterol trafficking that is part of the miR-33 and miR-27b target gene networks that contribute to the maintenance of cholesterol homeostasis.
Keywords: cholesterol; cholesterol homeostasis; lipids and lipoproteins; macrophages; microRNA.
© 2016 American Heart Association, Inc.
Figures
Figure 1. OSBPL6 is a novel miR-33 and miR-27b target gene
A) Relative hepatic expression of OSBPL6, OSBPL1 and ABCA1 mRNA in African green monkeys on chow diet treated with control miR or anti-miR-33 oligonucleotides for 4 wks (n=6/group). B, F) Predicted binding sites for miR-33a/b (B) or miR-27a/b (F) in the 3’UTR of human wild type (WT) OSBPL6 and with the binding site mutated (MUT) using RNA-Hybrid. C, G) Activity of WT or mutant OSBPL6 3’UTR-luciferase reporter constructs in HEK293 cells transfected with miR-33 mimics (C) or miR-27b mimics (G). D, H) Relative expression of OSBPL6 and OSBPL1 mRNA determined by qPCR in THP-1 macrophages transfected with miR-33 mimics or inhibitors (D) or miR-27b mimics or inhibitors (G). C-D,G,H Data are expressed as mean ± SD and are representative of three independent experiments. *P < 0.05, **P < 0.01 compared to control treatment. E) Expression of Osbpl6 mRNA in plaque macrophages isolated from Ldlr−/− mice fed a Western diet (14 wk) and treated with control miR or anti-miR-33 oligonucleotides for 4 wks (n=4/group). Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared to control.
Figure 2. OSBPL6 mRNA is induced in response to cellular and dietary cholesterol
Relative expression of OSBPL6, OSBPL1 and ABCA1 mRNA by qPCR analysis in A) THP1 macrophages treated with acLDL for 24 hours; B) Livers of Ldlr−/− mice (n=5) fed a chow or a Western diet for 14 weeks; C) Livers of African green monkeys (n=5/group) fed a chow or high-fat/high-cholesterol (HFHC) diet for 10 weeks. Data are expressed as mean ± SD. Data in (A) are representative of three independent experiments. *P < 0.05, **P < 0.01 compared to untreated group or chow diet.
Figure 3. OSBPL6 expression is regulated by the Liver X Receptor (LXR)
A) LXR response element (LXRE) in the promoter of human OSBPL6 as predicted by the JASPAR database. B) Relative enrichment of OSBPL6 and ABCA1 in LXRα chromatin IP from THP-1 cells treated with 10 μM LXRα agonist (T0901317). Data are normalized to control IgG. C) Activity of the wild type (WT) or LXRE-mutant OSBPL6 promoter-luciferase reporter in HEK293T cells treated with 10 μM T0901317 or DMSO. (D-F) Relative expression of OSBPL6, OSBPL1 and ABCA1 mRNA determined by qPCR in (D) THP-1 cells or (E) HepG2 cells treated with 10 μM T0901317 or vehicle control (DMSO) or (F) bone marrow derived macrophages from wild-type (WT) and Lxr−/− mice treated with 50 μg/mL acLDL or untreated. Data are expressed as mean ± SD and are representative of three independent experiments. *P < 0.05, **P < 0.01 compared to control treatment.
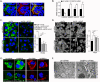
Figure 4. ORP6 localizes to early endosomes, lysosomes, and the ER
Immunofluorescence staining for FLAG (ORP6) and the indicated organelle markers (PM: plasma membrane, EE: early endosome, LE: late endosome, ER: endoplasmic reticulum, ERC: endocytic recycling compartment) in HEK293 cells transfected with FLAG-tagged ORP6. Co-localization is shown in the merged images as yellow and is indicated by arrows.
Figure 5. ORP6 regulates cholesterol trafficking
A) Co-localization of ORP6 and VAPA in HEK cells co-expressing FLAG-ORP6 and VAPA-GFP. B) Gene expression in THP-1 macrophages treated with control or OSBPL6 siRNA. Data are mean ± SEM of one experiment representative of three independent experiments. #P ≤ 0.1, *P < 0.05, **P < 0.005. C) BODIPY (neutral lipid) and D) Filipin (free cholesterol) staining in THP-1 cells transfected with control or OSBPL6 targeted siRNAs and loaded with acLDL (50 μg/mL) in the presence or absence of an inhibitor of acyl-CoA cholesterol acyltransferase (ACATi) to prevent the esterification of cholesterol in the ER. Quantification is shown at right; fluorescence images from an average of nine different fields were quantified by averaging the mean fluorescence intensity from an average of 70 cells (C) or 45 cells (D) selected randomly from each field by using image J. Data are the mean ± SEM of two independent experiments. *P < 0.05, **P < 0.005 (ANOVA). E) THP-1 macrophages were incubated with DiI-labeled LDL or AcLDL (10μg/mL) in the presence or absence of unlabeled lipoprotein in 5X excess (50μg/mL) for 4h to visualize internalized lipoproteins in cells treated with control or OSBPL6 siRNA. F) Electron microscopy of THP-1 macrophages treated with control or OSBPL6 siRNA and loaded with AcLDL (50μg/mL) for 24h.
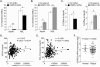
Figure 6. OSBPL6 regulates cholesterol efflux, is correlated with plasma levels of HDL and apoAI and its expression is reduced in atherosclerosis
A-C) Cholesterol efflux to apoAI or HDL by THP-1 cells (A-B) or to apoAI by HepG2 cells (C) that are transfected with a siRNA against OSBPL6 or control siRNA or an expression plasmid for OSBPL6. Dot plots showing direct relationship between human hepatic expression of OSBPL6 and plasma (D) HDL and (E) APOAI levels. Data are expressed as mean ± SD and are representative of three independent experiments. *P < 0.05, **P < 0.01 compared to control treatment. F) OSBPL6 mRNA expression (log2) in healthy arteries (Normal) or carotids from patients with atherosclerosis (Plaque) from the Biobank of Karolinska Endarterectomies (BiKE).
Similar articles
- MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis.
Meiler S, Baumer Y, Toulmin E, Seng K, Boisvert WA. Meiler S, et al. Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):323-31. doi: 10.1161/ATVBAHA.114.304878. Epub 2014 Dec 18. Arterioscler Thromb Vasc Biol. 2015. PMID: 25524771 Free PMC article. - Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144.
Ramírez CM, Rotllan N, Vlassov AV, Dávalos A, Li M, Goedeke L, Aranda JF, Cirera-Salinas D, Araldi E, Salerno A, Wanschel A, Zavadil J, Castrillo A, Kim J, Suárez Y, Fernández-Hernando C. Ramírez CM, et al. Circ Res. 2013 Jun 7;112(12):1592-601. doi: 10.1161/CIRCRESAHA.112.300626. Epub 2013 Mar 21. Circ Res. 2013. PMID: 23519695 Free PMC article. - miR-27b inhibits LDLR and ABCA1 expression but does not influence plasma and hepatic lipid levels in mice.
Goedeke L, Rotllan N, Ramírez CM, Aranda JF, Canfrán-Duque A, Araldi E, Fernández-Hernando A, Langhi C, de Cabo R, Baldán Á, Suárez Y, Fernández-Hernando C. Goedeke L, et al. Atherosclerosis. 2015 Dec;243(2):499-509. doi: 10.1016/j.atherosclerosis.2015.09.033. Atherosclerosis. 2015. PMID: 26520906 Free PMC article. - microRNAs in lipoprotein metabolism and cardiometabolic disorders.
Rotllan N, Price N, Pati P, Goedeke L, Fernández-Hernando C. Rotllan N, et al. Atherosclerosis. 2016 Mar;246:352-60. doi: 10.1016/j.atherosclerosis.2016.01.025. Epub 2016 Jan 18. Atherosclerosis. 2016. PMID: 26828754 Free PMC article. Review. - Foam cells in atherosclerosis.
Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Yu XH, et al. Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
Cited by
- Intracellular and Plasma Membrane Events in Cholesterol Transport and Homeostasis.
Litvinov DY, Savushkin EV, Dergunov AD. Litvinov DY, et al. J Lipids. 2018 Aug 6;2018:3965054. doi: 10.1155/2018/3965054. eCollection 2018. J Lipids. 2018. PMID: 30174957 Free PMC article. Review. - The miR-23-27-24 cluster: an emerging target in NAFLD pathogenesis.
Ru L, Wang XM, Niu JQ. Ru L, et al. Acta Pharmacol Sin. 2022 May;43(5):1167-1179. doi: 10.1038/s41401-021-00819-w. Epub 2021 Dec 10. Acta Pharmacol Sin. 2022. PMID: 34893685 Free PMC article. Review. - Regulation of macrophage immunometabolism in atherosclerosis.
Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Koelwyn GJ, et al. Nat Immunol. 2018 Jun;19(6):526-537. doi: 10.1038/s41590-018-0113-3. Epub 2018 May 18. Nat Immunol. 2018. PMID: 29777212 Free PMC article. Review. - MicroRNA-33 Inhibits Adaptive Thermogenesis and Adipose Tissue Beiging.
Afonso MS, Verma N, van Solingen C, Cyr Y, Sharma M, Perie L, Corr EM, Schlegel M, Shanley LC, Peled D, Yoo JY, Schmidt AM, Mueller E, Moore KJ. Afonso MS, et al. Arterioscler Thromb Vasc Biol. 2021 Apr;41(4):1360-1373. doi: 10.1161/ATVBAHA.120.315798. Epub 2021 Mar 4. Arterioscler Thromb Vasc Biol. 2021. PMID: 33657886 Free PMC article. - Bis(monoacylglycero)phosphate, an important actor in the host endocytic machinery hijacked by SARS-CoV-2 and related viruses.
Luquain-Costaz C, Rabia M, Hullin-Matsuda F, Delton I. Luquain-Costaz C, et al. Biochimie. 2020 Dec;179:247-256. doi: 10.1016/j.biochi.2020.10.018. Epub 2020 Nov 5. Biochimie. 2020. PMID: 33159981 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- R01 HL117226/HL/NHLBI NIH HHS/United States
- R01 HL119047/HL/NHLBI NIH HHS/United States
- T32 HL098129/HL/NHLBI NIH HHS/United States
- R01 HL111932/HL/NHLBI NIH HHS/United States
- R01 HL108182/HL/NHLBI NIH HHS/United States
- CIHR/Canada
- T32 AI007180/AI/NIAID NIH HHS/United States
- R00 HL088528/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases