Sex-specific IL-6-associated signaling activation in ozone-induced lung inflammation - PubMed (original) (raw)
Sex-specific IL-6-associated signaling activation in ozone-induced lung inflammation
Vikas Mishra et al. Biol Sex Differ. 2016.
Abstract
Background: Acute ozone (O3) exposure has known deleterious effects on the respiratory system and has been linked with respiratory disease and infection. Inflammatory lung disease induced by air pollution has demonstrated greater severity and poorer prognosis in women vs. men. Both severe damage to the bronchial-alveolar epithelium and malfunctioning of bronchial-blood barrier have been largely attributed to the pathobiology of O3-induced inflammatory response, but the associated mechanisms in the male and female lung remain unknown.
Methods: Here, we investigated sex-based differential regulation of lung interleukin-6 (IL-6) and its downstream signaling pathways JAK2/STAT3 and AKT1/NF-κB in response to O3 exposure in a mouse model. We exposed male and female mice (in different stages of the estrous cycle) to 2 ppm of O3 or filtered air (FA) for 3 h, and we harvested lung tissue for protein expression analysis by Western blot.
Results: We found significant up-regulation of IL-6 and IL-6R in females and IL-6 in males in response to O3 vs. FA. Ozone exposure induced a significant increase in STAT3-Y705 phosphorylation in both females and males. Males exposed to O3 had decreased levels of JAK2, but increased JAK2 (Y1007+Y1008) phosphorylation, while females exposed to O3 showed significant up-regulation of both proteins. Both NF-κB (p105/p50) and AKT1 protein levels were significantly increased only in females exposed to O3. In addition, females exposed to O3 during proestrus displayed increased expression of selected genes when compared to females exposed to O3 in other estrous cycle stages.
Conclusions: Together, our observations indicate a sex-based and estrous cycle-dependent differential lung inflammatory response to O3 and involvement of two converging JAK2/STAT3 and AKT1/NF-κB pathways. To our knowledge, this is the first study specifically addressing the impact of the estrous cycle in O3-associated lung inflammatory pathways.
Keywords: Estrous cycle; Gender differences; Inflammation; Lung disease; Oxidative stress.
Figures
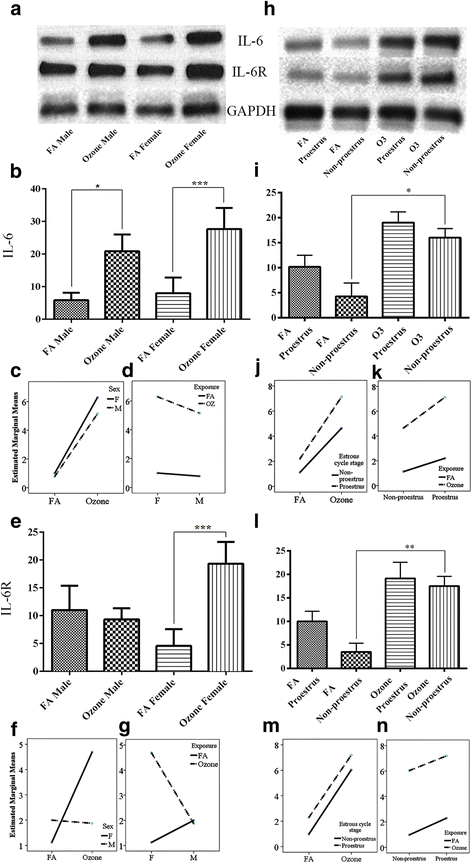
Fig. 1
IL6 and IL6R expression and effect of ozone exposure. Left panels: a Representative Western blot images of IL6 and IL6R expression in males and females with filter air and O3 exposure; univariate analysis of IL6 (b) and IL6R (e), expression in males and females, with FA and O3 exposure; two-way ANOVA interaction effect of sex (c) and exposure (d), for IL6 expression and sex (f) and exposure (g), for IL6R expression. Right panels: h Representative Western blot images of IL6 and IL6R expression in estrous cycle stages of females, with filter air and O3 exposure; univariate analysis of IL6 (i) and IL6R (l), expression in estrous cycle stages of females, with FA and O3 exposure. Two-way ANOVA interaction effect of exposure (j) and estrous cycle stages (k), for IL6 expression and exposure (m) and estrous cycle stages (n), for IL6R expression. Univariate analysis data expressed as Ranks-Kruskal-Wallis test of densitometric analysis; the values are depicted as mean with SD, where *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 are the levels of statistical significance compared to controls (n = 6–8 per group). Two-way ANOVA for IL6 and IL6R analysis is given in Tables 1 and 3, respectively
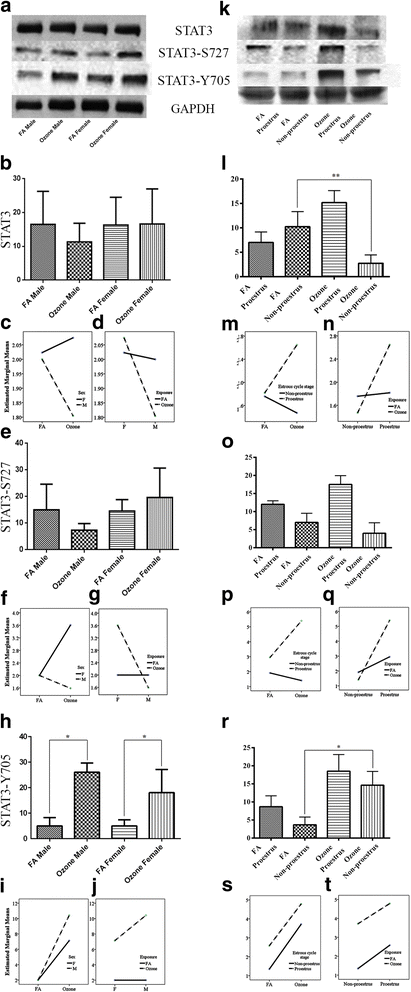
Fig. 2
STAT3, STAT3 Serine 727, and STAT3 Tyrosine 705 phosphorylation and effect of ozone exposure. Left panels: a Representative Western blot images of STAT3, STAT3 Serine 727 and STAT3 Tyrosine 705 phosphorylation expression in males and females with filter air and O3 exposure; univariate analysis of STAT3 (b), STAT3 Serine 727 (e), and STAT3 Tyrosine 705 (h), expression in males and females, with FA and O3 exposure; Two-way ANOVA interaction effect of sex (c) and exposure (d), for STAT3 expression, sex (f) and exposure (g), for STAT3 Serine 727 expression and sex (i) and exposure (j), for STAT3 Tyrosine 705 expression. Right panels: k Representative Western blot images of STAT3, STAT3 Serine 727, and STAT3 Tyrosine 705 phosphorylation expression in estrous cycle stages of females, with filter air and O3 exposure; univariate analysis of STAT3 (l), STAT3 Serine 727 (o), and STAT3 Tyrosine 705 (r), expression in estrous cycle stages of females, with FA and O3 exposure. Two-way ANOVA interaction effect of exposure (m) and estrous cycle stages (n), for STAT3 expression, exposure (p) and estrous cycle stages (q), for STAT3 Serine 727 expression, and exposure (s) and estrous cycle stages (t), for STAT3 Tyrosine 705 expression. Univariate analysis data expressed as Ranks-Kruskal-Wallis test of densitometric analysis; the values are depicted as mean with SD, where *p ≤ 0.05 and **p ≤ 0.01 are the levels of statistical significance compared to controls (n = 6–8 per group). Two-way ANOVA for STAT3, STAT3 Serine 727, and STAT3 Tyrosine 705 phosphorylation expression analysis is given in Tables 4, 5, and 6, respectively
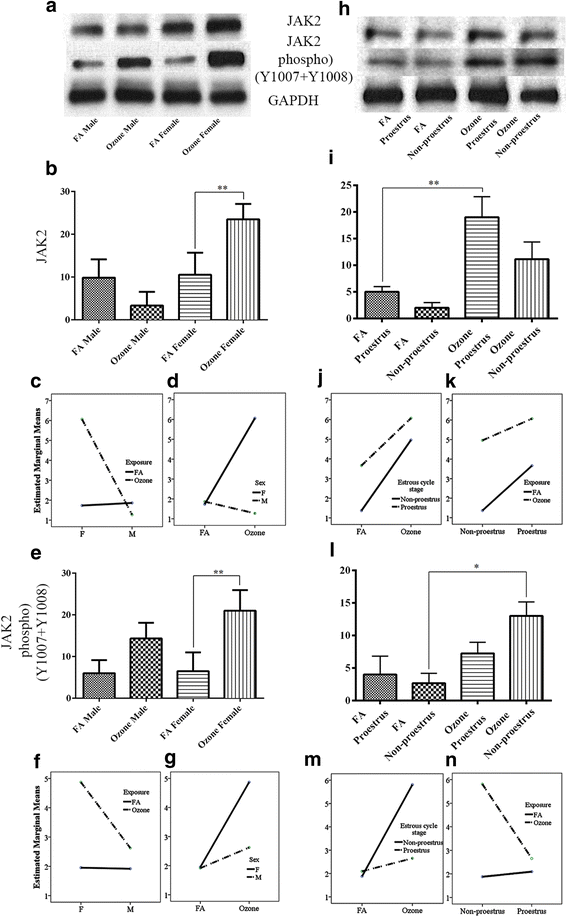
Fig. 3
JAK2 and JAK2 phosphorylated (Y1007+Y1008) expression and effect of ozone exposure. Left panels: a Representative Western blot images of JAK2 and JAK2 phosphorylated (Y1007+Y1008) expression in males and females with filter air and O3 exposure; univariate analysis of JAK2 (b) and JAK2 (e) phosphorylated (Y1007+Y1008), expression in males and females, with FA and O3 exposure; two-way ANOVA interaction effect of sex (c) and exposure (d), for JAK2 expression and sex (f) and exposure (g), for JAK2 phosphorylated (Y1007+Y1008) expression. Right panels: h Representative Western blot images of JAK2 and JAK2 phosphorylated (Y1007+Y1008) expression in estrous cycle stages of females, with filter air and O3 exposure; univariate analysis of i JAK2 and l JAK2 phosphorylated (Y1007+Y1008), expression in estrous cycle stages of females, with FA and O3 exposure. Two-way ANOVA interaction effect of exposure (j) and estrous cycle stages (k), for JAK2 expression and exposure (m) and estrous cycle stages (n) and for JAK2 phosphorylated (Y1007+Y1008) expression. Univariate analysis data expressed as Ranks-Kruskal-Wallis test of densitometric analysis; the values are depicted as mean with SD, where *p ≤ 0.05 and **p ≤ 0.01 are the levels of statistical significance compared to controls (n = 6–8 per group). Two-way ANOVA for JAK2 and JAK2 phosphorylated (Y1007+Y1008) analysis is given in Tables 7 and 8, respectively
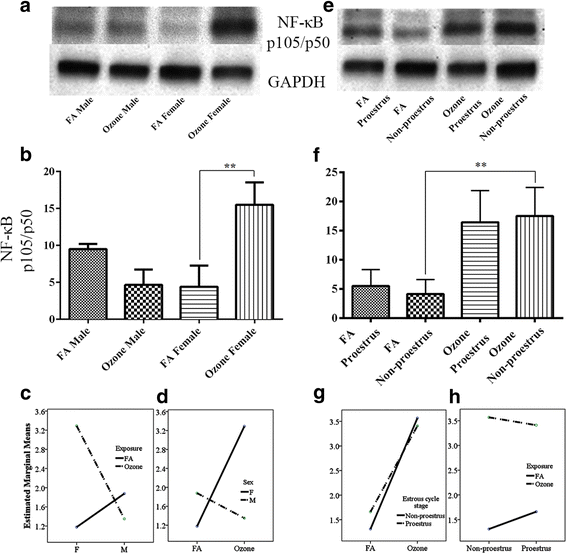
Fig. 4
NF-κB (p105/p50) expression and effect of ozone exposure. Left panel: a Representative Western blot images of NF-κB (p105/p50) expression in males and females with filter air and O3 exposure; b univariate analysis of NF-κB (p105/p50) expression in males and females with FA and O3 exposure; two-way ANOVA interaction effect of sex (c) and exposure (d) for NF-κB (p105/p50) expression. Right panel: e Representative Western blot images of NF-κB (p105/p50) expression in estrous cycle stages of females, with filter air and O3 exposure; f univariate analysis of NF-κB (p105/p50) expression in estrous cycle stages of females with FA and O3 exposure. Two-way ANOVA interaction effect of exposure (g) and estrous cycle stages (h) for NF-κB (p105/p50) expression. Univariate analysis data expressed as Ranks-Kruskal-Wallis test of densitometric analysis; the values are depicted as mean with SD, where **p ≤ 0.01 is the level of statistical significance compared to controls (n = 6–8 per group). Two-way ANOVA for NF-κB (p105/p50) expression analysis is given in Table 9
Fig. 5
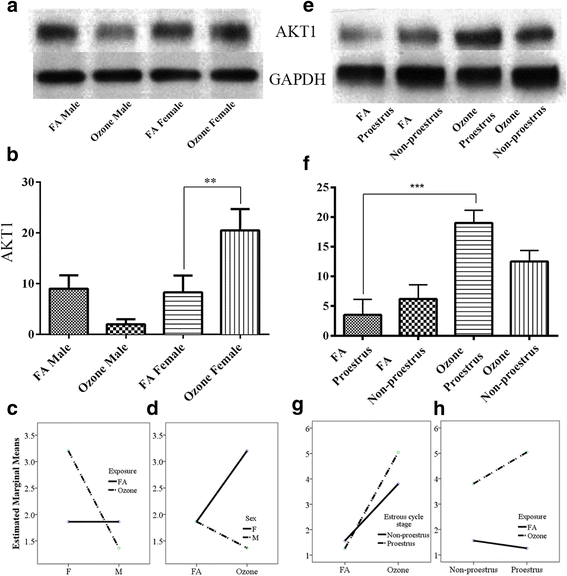
AKT1 expression and effect of ozone exposure. Left panel: a Representative Western blot images of AKT1 expression in males and females with filter air and O3 exposure; b univariate analysis of AKT1 expression in males and females with FA and O3 exposure; two-way ANOVA interaction effect of sex (c) and exposure (d) for AKT1 expression. Right panels: e Representative Western blot images of AKT1 expression in estrous cycle stages of females, with filter air and O3 exposure; f univariate analysis of AKT1 expression in estrous cycle stages of females with FA and O3 exposure. Two-way ANOVA interaction effect of exposure (g) and estrous cycle stages (h) for AKT1 expression. Univariate analysis data expressed as Ranks-Kruskal-Wallis test of densitometric analysis; the values are depicted as mean with SD where **p ≤ 0.01 and ***p ≤ 0.001 are the levels of statistical significance compared to controls (n = 6–8 per group). Two-way ANOVA for AKT1 expression analysis is given in Table 10
Fig. 6
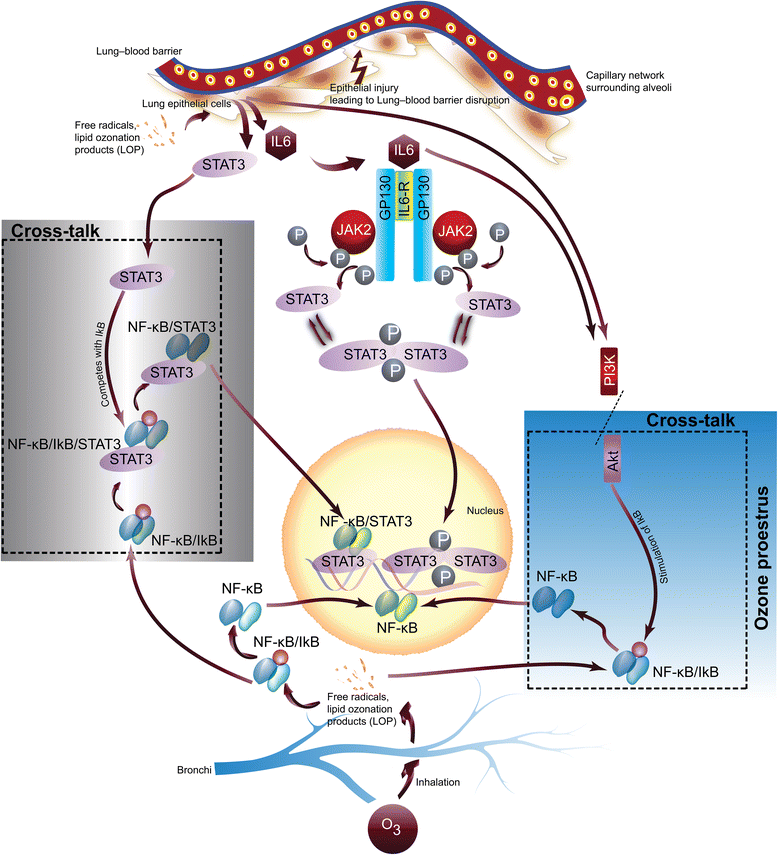
Schematic of ozone-induced lung inflammation and role of IL-6 signaling pathway: ozone-associated lung inflammation is primarily driven by lipid ozonation products (LOP) and generation of free radicals primarily in lung epithelial cells. LOP and free radicals can further mediate activation of downstream biochemical events and cascades of secondary cellular responses leading to the inflammatory damage. The production of free radicals and LOP can trigger (i) increased expression of IL-6 and STAT3 and (ii) activation of NF-κB and (iii) activation of PI3K/AKT pathway
Similar articles
- Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D.
Kierstein S, Poulain FR, Cao Y, Grous M, Mathias R, Kierstein G, Beers MF, Salmon M, Panettieri RA Jr, Haczku A. Kierstein S, et al. Respir Res. 2006 Jun 1;7(1):85. doi: 10.1186/1465-9921-7-85. Respir Res. 2006. PMID: 16740162 Free PMC article. - Mechanisms of response to ozone exposure: the role of mast cells in mice.
Kleeberger SR, Longphre M, Tankersley CG. Kleeberger SR, et al. Res Rep Health Eff Inst. 1999 Apr;(85):1-30; discussion 31-6. Res Rep Health Eff Inst. 1999. PMID: 10349676 - Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications.
Bhalla DK. Bhalla DK. J Toxicol Environ Health B Crit Rev. 1999 Jan-Mar;2(1):31-86. doi: 10.1080/109374099281232. J Toxicol Environ Health B Crit Rev. 1999. PMID: 10081525 Review. - Differential Susceptibility to Infectious Respiratory Diseases between Males and Females Linked to Sex-Specific Innate Immune Inflammatory Response.
Chamekh M, Deny M, Romano M, Lefèvre N, Corazza F, Duchateau J, Casimir G. Chamekh M, et al. Front Immunol. 2017 Dec 13;8:1806. doi: 10.3389/fimmu.2017.01806. eCollection 2017. Front Immunol. 2017. PMID: 29321783 Free PMC article. Review.
Cited by
- Ozone-induced changes in the murine lung extracellular vesicle small RNA landscape.
Smith GJ, Tovar A, Kanke M, Wang Y, Deshane JS, Sethupathy P, Kelada SNP. Smith GJ, et al. Physiol Rep. 2021 Sep;9(18):e15054. doi: 10.14814/phy2.15054. Physiol Rep. 2021. PMID: 34558223 Free PMC article. - Microvesicle-Derived miRNAs Regulate Proinflammatory Macrophage Activation in the Lung Following Ozone Exposure.
Carnino JM, Lee H, Smith LC, Sunil VR, Rancourt RC, Vayas K, Cervelli J, Kwok ZH, Ni K, Laskin JD, Jin Y, Laskin DL. Carnino JM, et al. Toxicol Sci. 2022 Apr 26;187(1):162-174. doi: 10.1093/toxsci/kfac025. Toxicol Sci. 2022. PMID: 35201360 Free PMC article. - Integrative analysis reveals mouse strain-dependent responses to acute ozone exposure associated with airway macrophage transcriptional activity.
Tovar A, Crouse WL, Smith GJ, Thomas JM, Keith BP, McFadden KM, Moran TP, Furey TS, Kelada SNP. Tovar A, et al. Am J Physiol Lung Cell Mol Physiol. 2022 Jan 1;322(1):L33-L49. doi: 10.1152/ajplung.00237.2021. Epub 2021 Nov 10. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 34755540 Free PMC article. - Cytokine expression in patients with interstitial lung disease in primary Sjogren's syndrome and its clinical significance.
Yang H. Yang H. Am J Transl Res. 2021 Jul 15;13(7):8391-8396. eCollection 2021. Am J Transl Res. 2021. PMID: 34377333 Free PMC article. - Sex Modifies Acute Ozone-Mediated Airway Physiologic Responses.
Birukova A, Cyphert-Daly J, Cumming RI, Yu YR, Gowdy KM, Que LG, Tighe RM. Birukova A, et al. Toxicol Sci. 2019 Jun 1;169(2):499-510. doi: 10.1093/toxsci/kfz056. Toxicol Sci. 2019. PMID: 30825310 Free PMC article.
References
- Organization WH . Health aspects of air pollution with particulate matter, ozone and nitrogen dioxide. Bonn, Germany: Report on a WHO working group; 2003. pp. 13–5.
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous