Arterial Remodeling in B-Type Natriuretic Peptide Knock-Out Females - PubMed (original) (raw)
Arterial Remodeling in B-Type Natriuretic Peptide Knock-Out Females
Sara J Holditch et al. Sci Rep. 2016.
Abstract
Sexual dimorphisms are recognized in cardiovascular conditions such as hypertension, stroke, thrombosis and vasculitis. B-type natriuretic peptide (BNP) is a guanylyl cyclase A (GC-A) agonist. The anti-hypertensive, vasodilatory, anti-fibrotic, and anti-hypertrophic properties of BNP are well established in male animal models. Although circulating BNP levels are higher in women, when compared to age-matched men, the cardiovascular protective propensity of BNP in females is poorly understood. We assessed the cardiovascular consequences of BNP deletion in genetically null (Nppb-/-) female rat lines. Throughout the study, blood pressure (BP) remained uninfluenced by genotype, and cardiorenal consequences of BNP knock out remained minor. Unexpectedly, approximately 60% of Nppb-/- females developed mesenteric polyarteritis-nodosa (PAN)-like vasculitis in their life span, some as early as 4 months of age. Mesenteric lesions involved intense arterial remodeling, progressive inflammation, occluded lumens, and less frequently intestinal necrosis and multiple visceral arterial aneurysms. Cumulative pathologies resulted in a significant decline in survival of the Nppb-/- female. This study highlights BNP's vasoprotective propensity, bringing to light a possible sex specific difference in the cardiovascular protection provided by BNP. Defects in the BNP/GC-A/cGMP pathway may play a role in arteriopathies in women, while GC-A agonists may provide effective therapy for arteritis.
Figures
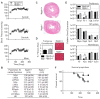
Figure 1. Preserved cardiac function in aged BNP knockout females.
(A) Noninvasive systolic, diastolic, and mean blood pressure measurements in Nppb−/− (n = 14) and age-matched control Nppb+/+ (n = 6); measurements were initiated at 25 days of age and monitored bimonthly through study termination at 9 months. Genotypes were assayed at the same time-point, however due to similarity of the data sets, BP lines are graphed staggered for visual ease. (B) Echocardiographic assessment of cardiac remodeling of surviving genotypes were measured at nine months, Nppb−/− (n = 5), Nppb+/+ (n = 4). (C) Representative myocardial histology of Nppb−/− and age-matched Nppb+/+ (D) and quantification of Massons Trichrome Staining at nine months (n = 5, n = 4) Nppb−/− and Nppb+/+ respectively, with cardiac sections at 40× magnification. (E) RT-PCR quantification at three months of Profibrosis associated genes; Collagen type 1a1 (Col1a1), Fibronectin-1 (Fn1), Transforming growth factor-β (TGFβ), Tissue inhibitor metalloprotease-1 (Timp1), Natriuretic Peptide (NP) system associated genes; Atrial Natriuretic Peptide (Nppa), B-type Natriuretic Peptide (Nppb), Natriuretic Peptide Receptors 1 and 2 (Npr1, Npr2, respectively) and Hypertrophic Cardiomyopathy (HCM) associated genes; Transthyretin (Ttr), Alpha Cardiac Actin (Actc), Myosin heavy chain 7 (Myh7) and Tropomyosin 1 (Tpm1) transcripts, housekeeping gene Beta Actin corrected. Nppb−/− (n = 6) and age-matched control Nppb+/+ (n = 6). *P < 0.05 vs Nppb+/+ by t test, data represent the mean ± SEM. (F) Survival curve of Nppb−/− (n = 14) and age-matched control Nppb+/+ (n = 6) over nine months; survival curve compared by log-rank (Mantel-Cox) test.
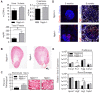
Figure 2. BNP deletion does not lead to marked renal pathology.
(A) Urine volume, calculated creatinine clearance, and urinary excreted protein at nine months were measured in Nppb−/− (n = 5) and age-matched control Nppb+/+ (n = 5). (B) Representative hematoxylin and eosin stained renal sections of Nppb−/− and Nppb+/+ (Black arrow indicates noticeably enlarged artery present in Nppb−/− section), and (C) quantification of renal pathology in Nppb−/− (n = 5) and Nppb+/+ (n = 4). (D) (Left) Representative microphotographs of immunofluorescent renal sections from 9 month Nppb−/− and 9 month Nppb+/+; anti-Desmin (Desmin, Red), and anti-Podocin (Blue). (Right) Representative Nppb−/− and Nppb+/+ glomeruli immunofluorescent imaging of anti-basic fibroblast growth factor 2 (Fgf2, Red) and anti-podocin (Blue), and nuclear counter stain, DAPI (White). (E) RT-PCR quantification of Collagen type 1a1 (Col1a1), Fibronectin-1 (Fn1), Transforming growth factor-β (TGFβ), Tissue inhibitor metalloprotease-1 (Timp1), Atrial Natriuretic Peptide (Nppa), B-type Natriuretic Peptide (Nppb), Natriuretic Peptide Receptors 1 and 2 (Npr1, Npr2, respectively) and Renal damage associated genes; Apolipoprotein E (Apoe1), Vascular endothelial growth factor (VegF), Jagged1 (Jag1) and Nephrosis 1 (Nphs1) transcripts are corrected to house keeping gene GAPDH. Nppb−/− (n = 6) and age-matched control Nppb+/+ (n = 6). *P < 0.05 vs Nppb+/+ by t test.
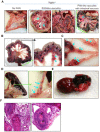
Figure 3. Polyarteritis like lesions, and arteriopathies in Nppb−/− females.
(A–C) Autopsy of moribund Nppb−/− females revealed mesenteric arterial nodes (black arrows) with partial intestinal necrosis (green bracket) and mesenteric arteries with multiple aneurysms (blue arrows). (D) Aneurysms found in splenic arteries and (E) epicardial hemorrhage in Nppb−/−. (F) HE staining demonstrated marked arterial wall thickening, inflammation of the affected arterial wall, and artery occlusion by possible fibrin plug or thrombi (black arrows).
Figure 4. Histology of the mesenteric arteries in Nppb−/− at 4 and 9 months.
(A) H&E analysis of progressive mesenteric arterial lesions in Nppb−/− . Mesenteric tissues obtained from animals of early and late symptom onset; 4 and 9 months in Nppb−/− vs 9 month Nppb+/+ (Control arteries highlighted by white arrows). (B) Factors involved in Nppb−/− mesenteric arterial remodeling: representative immunofluorescent microphotographs from 9 month Nppb−/− (n = 4), 4 month Nppb−/− (n = 3), and 9 month Nppb+/+(n = 3) sections, out of an average of 5 images per animal/section of mesenteric sections are shown. Specific antibodies include anti-Fgf2 (red), anti-Desmin (red), anti-α-SMA (green), anti-IL6 (green), anti-IL8 (green), and anti-CD45 (blue). Nuclei were counterstained by DAPI (white). SM; smooth muscle, ME: Mesentery, Control arteries highlighted by white arrows.
Figure 5. Coagulation component in aged Nppb−/− PAN.
(A–C) Serum analysis of Thrombin-Anti Thrombin Complex, Activated Partial Thromboplastin Time, and Citrate Prothrombin Time are shown. Nppb−/− (n = 5) and age-matched control Nppb+/+ (n = 5). *P < 0.05 vs Nppb+/+ by t test.
Similar articles
- B-Type Natriuretic Peptide Deletion Leads to Progressive Hypertension, Associated Organ Damage, and Reduced Survival: Novel Model for Human Hypertension.
Holditch SJ, Schreiber CA, Nini R, Tonne JM, Peng KW, Geurts A, Jacob HJ, Burnett JC, Cataliotti A, Ikeda Y. Holditch SJ, et al. Hypertension. 2015 Jul;66(1):199-210. doi: 10.1161/HYPERTENSIONAHA.115.05610. Epub 2015 May 11. Hypertension. 2015. PMID: 26063669 Free PMC article. - Pro-Atrial Natriuretic Peptide: A Novel Guanylyl Cyclase-A Receptor Activator That Goes Beyond Atrial and B-Type Natriuretic Peptides.
Ichiki T, Huntley BK, Sangaralingham SJ, Burnett JC Jr. Ichiki T, et al. JACC Heart Fail. 2015 Sep;3(9):715-23. doi: 10.1016/j.jchf.2015.03.015. JACC Heart Fail. 2015. PMID: 26362447 Free PMC article. - Natriuretic peptides buffer renin-dependent hypertension.
Demerath T, Staffel J, Schreiber A, Valletta D, Schweda F. Demerath T, et al. Am J Physiol Renal Physiol. 2014 Jun 15;306(12):F1489-98. doi: 10.1152/ajprenal.00668.2013. Epub 2014 Apr 9. Am J Physiol Renal Physiol. 2014. PMID: 24717731 - Biochemistry and physiology of the natriuretic peptide receptor guanylyl cyclases.
Tremblay J, Desjardins R, Hum D, Gutkowska J, Hamet P. Tremblay J, et al. Mol Cell Biochem. 2002 Jan;230(1-2):31-47. Mol Cell Biochem. 2002. PMID: 11952095 Review. - B-type Natriuretic Peptide circulating forms: Analytical and bioactivity issues.
Yandle TG, Richards AM. Yandle TG, et al. Clin Chim Acta. 2015 Aug 25;448:195-205. doi: 10.1016/j.cca.2015.07.004. Epub 2015 Jul 6. Clin Chim Acta. 2015. PMID: 26160054 Review.
Cited by
- Inflammation and Circulating Natriuretic Peptide Levels.
Fish-Trotter H, Ferguson JF, Patel N, Arora P, Allen NB, Bachmann KN, Daniels LB, Reilly MP, Lima JAC, Wang TJ, Gupta DK. Fish-Trotter H, et al. Circ Heart Fail. 2020 Jul;13(7):e006570. doi: 10.1161/CIRCHEARTFAILURE.119.006570. Epub 2020 Jun 8. Circ Heart Fail. 2020. PMID: 32507024 Free PMC article. - Guanylyl cyclase/natriuretic peptide receptor-A: Identification, molecular characterization, and physiological genomics.
Pandey KN. Pandey KN. Front Mol Neurosci. 2023 Jan 4;15:1076799. doi: 10.3389/fnmol.2022.1076799. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36683859 Free PMC article. Review. - The consequences of increased 4E-BP1 in polycystic kidney disease.
Holditch SJ, Brown CN, Atwood DJ, Pokhrel D, Brown SE, Lombardi AM, Nguyen KN, Hill RC, Lanaspa M, Hopp K, Weiser-Evans MCM, Edelstein CL. Holditch SJ, et al. Hum Mol Genet. 2019 Dec 15;28(24):4132-4147. doi: 10.1093/hmg/ddz244. Hum Mol Genet. 2019. PMID: 31646342 Free PMC article. - The landscape of sex-differential transcriptome and its consequent selection in human adults.
Gershoni M, Pietrokovski S. Gershoni M, et al. BMC Biol. 2017 Feb 7;15(1):7. doi: 10.1186/s12915-017-0352-z. BMC Biol. 2017. PMID: 28173793 Free PMC article. - Interventions in the B-type natriuretic peptide signalling pathway as a means of controlling chronic itch.
Meng J, Chen W, Wang J. Meng J, et al. Br J Pharmacol. 2020 Mar;177(5):1025-1040. doi: 10.1111/bph.14952. Epub 2020 Feb 12. Br J Pharmacol. 2020. PMID: 31877230 Free PMC article. Review.
References
- Rosendaal F. R., Helmerhorst F. M. & Vandenbroucke J. P. Female hormones and thrombosis. Arterioscler Thromb Vasc Biol 22, 201–210 (2002). - PubMed
- Rossouw J. E. et al.. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama 288, 321–333 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous