A novel tumor-promoting mechanism of IL6 and the therapeutic efficacy of tocilizumab: Hypoxia-induced IL6 is a potent autophagy initiator in glioblastoma via the p-STAT3-MIR155-3p-CREBRF pathway - PubMed (original) (raw)
. 2016 Jul 2;12(7):1129-52.
doi: 10.1080/15548627.2016.1178446. Epub 2016 May 10.
Guang Yuan 3, Xing Guo 1 2, Qinglin Liu 1, Jinsen Zhang 1 2, Xiao Gao 1, Xiaofan Guo 1, Shugang Xu 1 4, Tong Li 1, Qianqian Shao 5, Shaofeng Yan 2, Gang Li 1 2
Affiliations
- PMID: 27163161
- PMCID: PMC4990999
- DOI: 10.1080/15548627.2016.1178446
A novel tumor-promoting mechanism of IL6 and the therapeutic efficacy of tocilizumab: Hypoxia-induced IL6 is a potent autophagy initiator in glioblastoma via the p-STAT3-MIR155-3p-CREBRF pathway
Hao Xue et al. Autophagy. 2016.
Abstract
Hypoxia induces protective autophagy in glioblastoma cells and new therapeutic avenues that target this process may improve the outcome for glioblastoma patients. Recent studies have suggested that the autophagic process is upregulated in glioblastomas in response to extensive hypoxia. Hypoxia also induces the upregulation of a specific set of proteins and microRNAs (miRNAs) in a variety of cell types. IL6 (interleukin 6), an inflammatory autocrine and paracrine cytokine that is overexpressed in glioblastoma, has been reported to be a biomarker for poor prognosis because of its tumor-promoting effects. Here, we describe a novel tumor-promoting mechanism of IL6, whereby hypoxia-induced IL6 acts as a potent initiator of autophagy in glioblastoma via the phosphorylated (p)-STAT3-MIR155-3p pathway. IL6 and p-STAT3 levels correlated with the abundance of autophagic cells and HIF1A levels in human glioma tissues and with the grade of human glioma, whereas inhibition of exogenous or endogenous IL6 repressed autophagy in glioblastoma cells in vitro. Knockdown of endogenous MIR155-3p inhibited IL6-induced autophagy, and enforced expression of MIR155-3p restored the anti-autophagic activity of IL6 inhibitors. We show that the hypoxia-IL6-p-STAT3-MIR155-3p-CREBRF-CREB3-ATG5 pathway plays a central role in malignant glioma progression, with blockade of the IL6 receptor by tocilizumab demonstrating a certain level of therapeutic efficacy in a xenograft model in vivo, especially in combination with temozolomide. Moreover, tocilizumab inhibits autophagy by promoting tumor apoptosis. Collectively, our findings provide new insight into the molecular mechanisms underlying hypoxia-induced glioma cell autophagy and point toward a possible efficacious adjuvant therapy for glioblastoma patients.
Keywords: ATG5; CREB3; CREBRF; IL6; STAT3; autophagy; glioblastoma; hypoxia; microRNAs; tocilizumab.
Figures
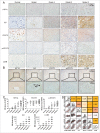
Figure 1.
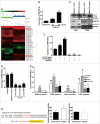
Levels of colocalized IL6, HIF1A, LC3B and p-STAT3 positively correlate with the WHO grade in human glioma tissues. (A) The expression of IL6, HIF1A, p-STAT3, and LC3B positively correlated with the WHO grade of gliomas. Ninety human gliomas and 3 normal brain tissue samples of different WHO grades were processed by immunohistochemistry. STAT3 levels were basically stable. (B) Immunohistochemical staining of continuous paraffin sections revealed the colocalization of IL6, p-STAT3, HIF1A, and LC3B proteins in the hypoxic area around the tumor vessels of a sample of high-grade glioma tissues (WHO III) (enlarged view of the lower right green box in Fig. S1). (C) Quantification of IHC staining of glioma tissues with different WHO grades. IRS, immunoreactive score. (D) Pearson's or Spearman's rho rank correlation tests of IL6, p-STAT3, HIF1A, LC3B and grades.
Figure 2.
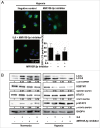
Hypoxia induces autophagy activation and upregulates IL6 in human glioma cells. (A) Hypoxia promotes GFP-LC3B translocation. pSELECT-GFP-LC3B transfection revealed LC3B puncta in U251 cells treated with hypoxia (1% O2, 5% CO2, and 94% N2 at 37°C) for 24 h. Cells were fixed and stained with DAPI for nuclear visualization. Representative images are shown. Scale bar: 50 μm. Quantitative analysis of GFP-LC3B puncta is shown in the right panel. At least 100 high-power fields were examined in each experimental group. The data shown are the mean ± s.d. of 4 independent experiments. *, P < 0.0001; 2-tailed t test. (B) Hypoxia induced LC3B conversion and SQSTM1 degradation in U251 and T98G cells. LC3B and SQSTM1 levels were examined by western blot analysis in GBM cells after hypoxia treatment (1% O2, 5% CO2, and 94% N2 at 37°C) for 12 and 24 h. GAPDH served as the loading control. (C) Western blot analysis showing that 3-methyladenine (3-MA) inhibited autophagy in U251 cells and treatment of normoxic and hypoxic cells with bafilomycin (BAF) blocked autophagic flux. (D) Hypoxia increased the secretion of IL6 in U251 and T98G cell culture supernatants, as revealed by ELISA. The data shown are the mean ± s.d. of 3 independent experiments. * and #, P < 0.001; 2-tailed t test.
Figure 3.
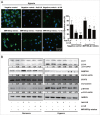
IL6 induces autophagy activation, and inhibition of IL6 represses autophagy in human glioma cells. (A) Exogenous IL6 promotes GFP-LC3B translocation. pSELECT-GFP-LC3B-transfected U251 cells treated with IL6 (20 ng/ml) for 24 h. Scale bar: 50 μm. Quantitative analysis of GFP-LC3B puncta is shown in the right panel. The data shown are the mean ± s.d. of 4 independent experiments. *, P < 0.0001; 2-tailed t test. (B) Exogenous IL6 induced LC3B conversion and STAT3 activation in U251 and T98G cells. LC3B, STAT3 and p-STAT3 levels were examined by western blot analysis in GBM cells after treatment with IL6 (20 ng/ml) for 0, 12, 24 h. GAPDH served as the loading control. (C) Images from transmission electron microscopy showing characteristic autophagosomes (arrows) in U251 cells after treatment with IL6 (20 ng/ml) for 24 h. N, nucleus. At least 50 cells were examined in each experimental group. The data shown are the mean ± s.d. of 3 independent experiments. *, P < 0.0001; 2-tailed t test. (D) Exogenous IL6 induces LC3B conversion and SQSTM1 degradation in GBM cells in a dose-dependent manner after a 24-h treatment. Inhibition of endogenous IL6 (E) and exogenous IL6 (F) represses LC3B conversion and SQSTM1 degradation in GBM cells as measured by western blot analysis. (G) Autophagic flux inhibition of IL6-treated U251 cells with bafilomycin A1 (BAF). ACTB served as the loading control.
Figure 4.
Activation of the IL6-p-STAT3 pathway is involved in hypoxia-induced autophagy in glioblastoma cells. (A) An antibody against exogenous IL6 inhibited GFP-LC3B translocation. pSELECT-GFP-LC3B-transfected U251 cells treated with IL6 (20 ng/ml) and an IL6 antibody (1 μg/ml) for 24 h. Scale bar: 50 μm. Quantitative analysis of GFP-LC3B puncta is shown in the right panel. The data shown are the mean ± s.d. of 4 independent experiments. * and #, P<0.001; one-way ANOVA. (B) An antibody against exogenous IL6 inhibited LC3B conversion and STAT3 activation in U251 and T98G cells. LC3B, STAT3 and p-STAT3 levels were examined by western blot analysis in GBM cells after treatment with IL6 (20 ng/ml) and an IL6 antibody (1 μg/ml) for 24 h. GAPDH served as the loading control. (C) An antibody against exogenous IL6 inhibited GFP-LC3B translocation in hypoxic U251 cells. pSELECT-GFP-LC3B-transfected U251 cells treated with IL6 antibody (1 μg/ml) for 24 h under hypoxic conditions. Scale bar: 50 μm. The quantitative analysis of GFP-LC3B puncta is shown in the right panel. The data shown are the mean ± s.d. of 4 independent experiments. *, P < 0.0001; 2-tailed t test. (D) An antibody against exogenous IL6 inhibited LC3B conversion and STAT3 activation in hypoxic U251 and T98G cells. LC3B, STAT3 and p-STAT3 levels were examined by western blot analysis after treatment of hypoxic GBM cells with an IL6 antibody (1 μg/ml) for 24 h. GAPDH served as the loading control.
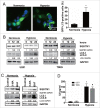
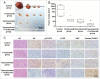
Figure 5.
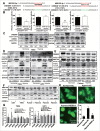
MIR155-3p is upregulated by hypoxia, and IL6 can induce autophagy in glioblastoma cells. (A) The miRCURY™ RNA expression array revealed 84 significantly differentially expressed miRNAs (partial data shown in Fig. 5A) between normoxic and hypoxic U251 cells. The hypoxic miRNA marker MIR210 and the target miRNA MIR155-3p are indicated. (B) The expression levels of MIR155-3p in hypoxic U251 cells (hypoxia treatment for 0, 12, and 24 h) were assessed by quantitative real-time PCR. The data shown are the mean ± s.d. of 5 independent experiments. *, P < 0.05; ***, P < 0.0001; one-way ANOVA. (C) _MIR155-3p_ overexpression induced LC3B conversion and SQSTM1 degradation in U251 and T98G cells at 48 h after _MIR155-3p_ mimic transfection, as shown by western blot analysis. GAPDH served as the loading control. (D) Exogenous IL6 upregulated and an antibody against exogenous IL6 inhibited the expression levels of _MIR155-3p_, as determined by quantitative real-time PCR. The data shown are the mean ± s.d. of 5 independent experiments. *, P < 0.01; ***, P < 0.0001; one-way ANOVA. (E) Knockdown of endogenous IL6 inhibited the expression of _MIR155-3p_, as determined by quantitative real-time PCR. (F) The specific p-STAT3 inhibitor Stattic inhibited the function of STAT3. (G) A “5′-TTTCCCCAAA-3′” p-STAT3-binding element is present at −731 in the _MIR155-3p_ promoter. Mutation of the p-STAT3-binding element eliminated the promoting effect of the IL6-p-STAT3 pathway on _MIR155-3p_ expression compared with the wild-type element. The data shown are the mean ± s.d. of 5 independent experiments. * and #, P < 0.01; ** and ##, P < 0.001; ***, ### and $, P < 0.0001; * by Student's t-test for Stattic groups versus IL6 groups, # by the Student t test for IL6 groups vs. DMSO control groups, and $ by Student t test for hypoxia groups versus normoxia groups.
Figure 6.
MIR155-3p knockdown antagonizes hypoxia-induced autophagy in human glioma cells. (A) The MIR155-3p inhibitor suppressed GFP-LC3B translocation. pSELECT-GFP-LC3B and MIR155-3p inhibitor-cotransfected U251 cells were treated with IL6 (20 ng/ml) for 24 h. Scale bar: 50 μm. The quantitative analysis of GFP-LC3B puncta is shown in the right panel. The data shown are the mean ± s.d. of 4 independent experiments. * and #, P < 0.001; one-way ANOVA. (B) MIR155-3p inhibition suppresses LC3B conversion and STAT3 activation in U251 and T98G cells. LC3B, SQSTM1, STAT3 and p-STAT3 levels were examined by western blot analysis in GBM cells after treatment with IL6 (20 ng/ml) for 24 h. GAPDH served as the loading control.
Figure 7.
MIR155-3p overexpression enhances hypoxia-induced autophagy and resists the effects of IL6 inhibition on human glioma cells. (A) MIR155-3p mimic promoted GFP-LC3B translocation. pSELECT-GFP-LC3B and MIR155-3p mimic-co-transfected U251 cells treated with the IL6 antibody (1 μg/ml) and siRNAs (Si IL6 321) for 24 h. Scale bar: 50 μm. Quantitative analysis of GFP-LC3B puncta is shown in the right panel. The data shown are the mean ± s.d. of 4 independent experiments. *, # and $, P < 0.01; **, P < 0.001; ***, P < 0.0001; one-way ANOVA. (B) The MIR155-3p mimic induced LC3B conversion and STAT3 activation in U251 and T98G cells. LC3B, SQSTM1, STAT3 and p-STAT3 levels were examined by western blot analysis. GAPDH served as the loading control.
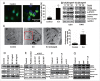
Figure 8.
MIR155-3p enhances hypoxia-induced autophagy by directly targeting the CREBRF-CREB3-ATG5 pathway in human glioma cells. (A) The best potential MIR155-3p target gene, CREBRF, in miRDB, and the 3′-UTR seed mutation. (B) The 3′-UTR seed mutation and 3′-UTR luciferase assays in U251 glioma cells. The luciferase activity in _MIR155-3p_-transfected cells decreased to approximately half of the activity observed using the control miRNA. (C) The inhibitory effect of MIR155-3p at the protein level of CREBRF and CREB3. We performed western blotting at 48 h after MIR155-3p inhibitor and mimic transfection into U251 and T98G cells and observed significantly decreased CREBRF protein levels in hypoxia-treated U251 and T98G cells. As CREBRF is a negative regulator of CREB3, the CREB3 level showed an opposite trend. (D) The CREB3 siRNA knockdown decreases autophagy levels and ATG5 protein levels of U251 and T98G cells. (E) and (F) The key pathway proteins in primary glioma cells by western blotting. (G) The CREB3-induced autophagy-related (ATG) genes in U251 and T98G cells by quantitative real-time PCR. (H) CREB3 siRNA inhibits GFP-LC3B translocation. pSELECT-GFP-LC3B-transfected U251 cells treated with IL6 (20 ng/ml) for 24 h. Scale bar: 50 μm. Quantitative analysis of GFP-LC3B puncta is shown in the right panel. The data shown are the mean ± s.d. of 4 independent experiments. * and #, P < 0.05; ** and ##, P < 0.01; 2-tailed t test. 155i, MIR155-3p inhibitor; 155m, MIR155-3p mimic; NC, negative control.
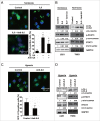
Figure 9.
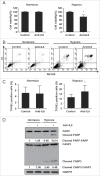
Inhibition of IL6 induces apoptosis in GBM cells. (A) A CCK-8 assay was performed to assess cell viability in hypoxia-treated U251 cells in the presence or absence of an IL6 antibody for 48 h. (B) and (C) ANXA5 FITC-PI and TUNEL staining assays were performed to examine the level of apoptosis in hypoxic GBM cells treated with an IL6 antibody (1 μg/ml). (D) Cleavage of PARP and CASP3 was induced by the IL6 antibody (1 μg/ml) in hypoxic GBM cells. PARP, cleaved PARP, CASP3 and cleaved CASP3 levels were examined by western blot analysis. GAPDH served as the loading control. The data are the mean ± s.d. *, P < 0.01 compared with the control group.
Figure 10.
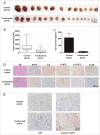
The IL6 monoclonal antibody tocilizumab inhibits the growth of GBM cells in vivo by inhibiting IL6-induced autophagy and promoting apoptosis. (A) and (B) Tocilizumab markedly inhibited tumor growth in U251 cell xenografts, as measured by tumor volume. *, P < 0.001 compared with the control group. (C) IL6 in the peripheral blood serum of the tocilizumab group was maintained at a lower level, as examined by ELISA, relative to the control group. *, P < 0.001 compared with the control group. (D) Tocilizumab reduced the expression of IL6, p-STAT3 and LC3B in all xenograft specimens, as evaluated by immunohistochemical staining. (E) Tocilizumab inhibited the proliferation and induced apoptosis in xenografts. The expression of KI67 and cleaved CASP3 in all xenograft specimens was examined by immunohistochemical staining.
Figure 11.
The IL6 monoclonal antibody tocilizumab increases the efficiency of temozolomide for GBM treatment in vivo by inhibiting IL6-induced autophagy and promoting apoptosis. (A) and (B) Tocilizumab and temozolomide combination therapy markedly inhibited tumor growth in U251 cell xenografts, as measured by tumor volume. *, P < 0.05 compared with the control group. #, P < 0.05 compared with the temozolomide group. (C) Tocilizumab reduced the expression of IL6, p-STAT3 and LC3B and induced apoptosis in temozolomide-treated xenograft specimens, as examined by immunohistochemical staining.
Figure 12.
Proposed model and hypothesis of the novel tumor-promoting mechanism of IL6 and the therapeutic efficacy of tocilizumab: hypoxia-induced IL6 is a potent autophagy initiator in glioblastoma via the p-STAT3-_MIR155-3p_-CREBRF pathway. The hypoxic regions around the tumor vessels (Fig. 1B) and hypoxia-induced IL6 trigger hypoxic glioma cell autophagy through the p-STAT3-_MIR155-3p_-CREBRF pathway. Because upregulated autophagy inhibits tumor apoptosis and promotes proliferation, we blocked the IL6 receptor using tocilizumab and significantly suppressed tumor growth by shifting from autophagy to apoptosis. TAM, tumor-associated macrophage.
Similar articles
- CREBRF is a potent tumor suppressor of glioblastoma by blocking hypoxia-induced autophagy via the CREB3/ATG5 pathway.
Xue H, Zhang J, Guo X, Wang J, Li J, Gao X, Guo X, Li T, Xu S, Zhang P, Liu Q, Li G. Xue H, et al. Int J Oncol. 2016 Aug;49(2):519-28. doi: 10.3892/ijo.2016.3576. Epub 2016 Jun 9. Int J Oncol. 2016. PMID: 27278737 - MiR224-3p inhibits hypoxia-induced autophagy by targeting autophagy-related genes in human glioblastoma cells.
Guo X, Xue H, Guo X, Gao X, Xu S, Yan S, Han X, Li T, Shen J, Li G. Guo X, et al. Oncotarget. 2015 Dec 8;6(39):41620-37. doi: 10.18632/oncotarget.5871. Oncotarget. 2015. PMID: 26536662 Free PMC article. - The HIF‑1α/miR‑224‑3p/ATG5 axis affects cell mobility and chemosensitivity by regulating hypoxia‑induced protective autophagy in glioblastoma and astrocytoma.
Huang S, Qi P, Zhang T, Li F, He X. Huang S, et al. Oncol Rep. 2019 Mar;41(3):1759-1768. doi: 10.3892/or.2018.6929. Epub 2018 Dec 13. Oncol Rep. 2019. PMID: 30569180 - The role of STAT3 in autophagy.
You L, Wang Z, Li H, Shou J, Jing Z, Xie J, Sui X, Pan H, Han W. You L, et al. Autophagy. 2015;11(5):729-39. doi: 10.1080/15548627.2015.1017192. Autophagy. 2015. PMID: 25951043 Free PMC article. Review. - Targeting strategies on miRNA-21 and PDCD4 for glioblastoma.
Wang G, Wang JJ, Tang HM, To SS. Wang G, et al. Arch Biochem Biophys. 2015 Aug 15;580:64-74. doi: 10.1016/j.abb.2015.07.001. Epub 2015 Jul 2. Arch Biochem Biophys. 2015. PMID: 26142886 Review.
Cited by
- An untapped window of opportunity for glioma: targeting therapy-induced senescence prior to recurrence.
Riviere-Cazaux C, Carlstrom LP, Neth BJ, Olson IE, Rajani K, Rahman M, Ikram S, Mansour MA, Mukherjee B, Warrington AE, Short SC, von Zglinicki T, Brown DA, Burma S, Tchkonia T, Schafer MJ, Baker DJ, Kizilbash SH, Kirkland JL, Burns TC. Riviere-Cazaux C, et al. NPJ Precis Oncol. 2023 Nov 29;7(1):126. doi: 10.1038/s41698-023-00476-8. NPJ Precis Oncol. 2023. PMID: 38030881 Free PMC article. Review. - SPI1-induced downregulation of FTO promotes GBM progression by regulating pri-miR-10a processing in an m6A-dependent manner.
Zhang S, Zhao S, Qi Y, Li B, Wang H, Pan Z, Xue H, Jin C, Qiu W, Chen Z, Guo Q, Fan Y, Xu J, Gao Z, Wang S, Guo X, Deng L, Ni S, Xue F, Wang J, Zhao R, Li G. Zhang S, et al. Mol Ther Nucleic Acids. 2022 Jan 1;27:699-717. doi: 10.1016/j.omtn.2021.12.035. eCollection 2022 Mar 8. Mol Ther Nucleic Acids. 2022. PMID: 35317283 Free PMC article. - miR766-3p and miR124-3p Dictate Drug Resistance and Clinical Outcome in HNSCC.
Shibata T, Cao DY, Dar TB, Ahmed F, Bhat SA, Veiras LC, Bernstein EA, Khan AA, Chaum M, Shiao SL, Tourtellotte WG, Giani JF, Bernstein KE, Cui X, Vail E, Khan Z. Shibata T, et al. Cancers (Basel). 2022 Oct 27;14(21):5273. doi: 10.3390/cancers14215273. Cancers (Basel). 2022. PMID: 36358691 Free PMC article. - Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury.
Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang C, Ge W, Wu J, Du P, Chen Y. Zhou M, et al. EBioMedicine. 2018 Sep;35:345-360. doi: 10.1016/j.ebiom.2018.08.035. Epub 2018 Aug 29. EBioMedicine. 2018. PMID: 30170968 Free PMC article. - Identifying PLAUR as a Pivotal Gene of Tumor Microenvironment and Regulating Mesenchymal Phenotype of Glioblastoma.
Fu Z, Chen Z, Ye J, Ji J, Ni W, Lin W, Lin H, Lu L, Zhu G, Xie Q, Yan F, Chen G, Liu F. Fu Z, et al. Cancers (Basel). 2024 Feb 19;16(4):840. doi: 10.3390/cancers16040840. Cancers (Basel). 2024. PMID: 38398231 Free PMC article.
References
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science 2004; 306:990-5; PMID:15528435; http://dx.doi.org/10.1126/science.1099993 - DOI - PMC - PubMed
- Thorburn A, Thamm DH, Gustafson DL. Autophagy and cancer therapy. Mol Pharmacol 2014; 85:830-8; PMID:24574520; http://dx.doi.org/10.1124/mol.114.091850 - DOI - PMC - PubMed
- Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res 2014; 74:647-51; PMID:24459182; http://dx.doi.org/10.1158/0008-5472.CAN-13-2966 - DOI - PubMed
- Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell 2013; 155:1216-9; PMID:24315093; http://dx.doi.org/10.1016/j.cell.2013.11.019 - DOI - PMC - PubMed
- Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol 2010; 32:431-6; PMID:20814791; http://dx.doi.org/10.1007/s00281-010-0220-1 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous