Nucleolar localization of Small G protein RhoA is associated with active RNA synthesis in human carcinoma HEp-2 cells - PubMed (original) (raw)
Nucleolar localization of Small G protein RhoA is associated with active RNA synthesis in human carcinoma HEp-2 cells
Yueying Li et al. Oncol Lett. 2016 Jun.
Abstract
Previous studies have demonstrated that the nuclear localization of ras homolog family member A (RhoA), with prominent concentration in the nucleolus, is a common feature in human cancer tissues and cancer cell lines. Although a previous study has demonstrated that the nuclear translocation of RhoA occurs via active transport, a process that occurs through importin α in a nuclear factor-κB-dependent manner, the mechanism, biological function and pathological meaning of the nucleolar residency of RhoA remain to be elucidated. As the cell nucleolus is the site of ribosome biosynthesis, the aim of the present study was to investigate the association between RNA synthesis and the nucleolar localization of RhoA, as well as the molecular mechanisms underlying the residency of RhoA in the nucleolus of HEp-2 (human larynx epithelial carcinoma) cells. Indirect immunofluorescence microscopy was used to evaluate the subcellular distribution of nuclear RhoA, and immunoblotting analysis was used to determine the total cellular protein level of RhoA. Consistent with the results of previous studies, untreated HEp-2 cells exhibited bright nucleolar staining, indicating an increased concentration of RhoA in the nucleoli. Treatment with actinomycin D for the inhibition of RNA synthesis caused a redistribution of RhoA from the nucleoli to the nucleoplasm with a speckled staining pattern. Immunoblotting revealed that neither the total cellular amount of RhoA nor the integrity of RhoA was affected by treatment with actinomycin D. In cells that were treated at a decreased concentration (0.05 mg/l) of actinomycin D, the redistribution of RhoA was reversible following the removal of the drug from the culture medium. However, this reversal was not observed at an increased drug concentration (1 mg/l). Overall, to the best of our knowledge, the results of the present study provide the first in situ evidence that the inhibition of RNA synthesis induces a redistribution of nucleolar RhoA to the nucleoplasm, and additionally suggest that the nucleolar residency of RhoA in HEp-2 cells may be associated with active RNA synthesis.
Keywords: RNA synthesis; actinomycin D; cell nucleus; human cancer cells; ras homolog family member A.
Figures
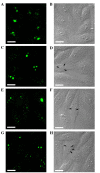
Figure 1.
Alteration of the intracellular localization of RhoA induced by actinomycin D in HEp-2 cells. (A) Indirect immunofluorescence revealed prominent nucleolar RhoA in untreated cells. (B) Corresponding cells were revealed in differential interference contrast pictures. Redistribution of nucleolar RhoA to nucleoplasmic speckles was observed in the cells treated with actinomycin D at the concentrations of (C) 0.05 mg/l, (D) differential interference contrast; (E) 0.10 mg/l, (F) differential interference contrast; and (G) 1.00 mg/l for 4 h, (H) differential interference contrast. Arrows represent the segregated nucleoli or nucleolus-like bodies. Scale bar, 10 µm. RhoA, ras homolog family member A.
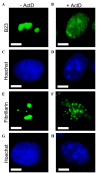
Figure 2.
Actinomycin D altered the subcellular location of nucleolar proteins B23 and fibrillarin in HEp-2 cells. Cells showed nucleolar localization of B23 (A) without treatment and (B) with 0.05 mg/l actinomycin D for 4 h. B23 translocated from the nucleoli to the nucleoplasm in a diffuse staining pattern. (C and D) Corresponding cell nuclei were shown by nuclear staining with Hoechst 33342. Cells demonstrated nucleolar localization of fibrillarin (E) without treatment and (F) with 0.05 mg/l actinomycin D for 4 h. Fibrillarin redistributed to small nucleoplasmic entities. (G and H) Corresponding cell nuclei were shown by nuclear staining with Hoechst 33342. Scale bar, 5 µm. B23, nucleolar phosphoprotein B23.
Figure 3.
Immunoblotting of RhoA protein amount in whole HEp-2 cell lysate. Total RhoA protein was detected as a single band at 21 kDa in untreated cells and cells treated with actinomycin D for 4 h at the concentration of 0.05 mg/l, 0.1 mg/l or 1 mg/l. Glyceraldehyde-3-phosphate dehydrogense was used as a loading control. RhoA, ras homolog family member A.
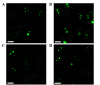
Figure 4.
Localization of ras homolog family member A in HEp-2 cells following the removal of actinomycin D. (A) Cells treated with actinomycin D at a concentration of 0.05 mg/l for 4 h. (B) Cells treated with actinomycin D at a concentration of 0.05 mg/l for 4 h, washed and incubated for 24 h in drug-free culture medium. (C) Cells treated with actinomycin D at a concentration of 1 mg/l for 1 h. (D) Cells treated with actinomycin D at a concentration of 1 mg/l for 1 h, washed and incubated for 27 h in drug-free culture medium. Scale bar, 10 µm.
Similar articles
- Altered subcellular distribution of nucleolar protein fibrillarin by actinomycin D in HEp-2 cells.
Chen M, Jiang P. Chen M, et al. Acta Pharmacol Sin. 2004 Jul;25(7):902-6. Acta Pharmacol Sin. 2004. PMID: 15210063 - Nuclear translocation of small G protein RhoA via active transportation in gastric cancer cells.
Xu J, Li Y, Yang X, Chen Y, Chen M. Xu J, et al. Oncol Rep. 2013 Oct;30(4):1878-82. doi: 10.3892/or.2013.2638. Epub 2013 Jul 25. Oncol Rep. 2013. PMID: 23900609 - Localization of ribosomal protein S1 in the granular component of the interphase nucleolus and its distribution during mitosis.
Hügle B, Hazan R, Scheer U, Franke WW. Hügle B, et al. J Cell Biol. 1985 Mar;100(3):873-86. doi: 10.1083/jcb.100.3.873. J Cell Biol. 1985. PMID: 3882724 Free PMC article. Review. - The nucleolus: a model for the organization of nuclear functions.
Hernandez-Verdun D. Hernandez-Verdun D. Histochem Cell Biol. 2006 Aug;126(2):135-48. doi: 10.1007/s00418-006-0212-3. Epub 2006 Jul 12. Histochem Cell Biol. 2006. PMID: 16835752 Review.
Cited by
- VPRBP Functions Downstream of the Androgen Receptor and OGT to Restrict p53 Activation in Prostate Cancer.
Poulose N, Forsythe N, Polonski A, Gregg G, Maguire S, Fuchs M, Minner S, Sauter G, McDade SS, Mills IG. Poulose N, et al. Mol Cancer Res. 2022 Jul 6;20(7):1047-1060. doi: 10.1158/1541-7786.MCR-21-0477. Mol Cancer Res. 2022. PMID: 35348747 Free PMC article. - CX-5461 causes nucleolar compaction, alteration of peri- and intranucleolar chromatin arrangement, an increase in both heterochromatin and DNA damage response.
Snyers L, Laffer S, Löhnert R, Weipoltshammer K, Schöfer C. Snyers L, et al. Sci Rep. 2022 Aug 17;12(1):13972. doi: 10.1038/s41598-022-17923-4. Sci Rep. 2022. PMID: 35978024 Free PMC article.
References
- Hernandez-Verdun D. The nucleolus today. J Cell Sci. 1991;99:465–471. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials