Liver Necrosis and Lethal Systemic Inflammation in a Murine Model of Rickettsia typhi Infection: Role of Neutrophils, Macrophages and NK Cells - PubMed (original) (raw)
Liver Necrosis and Lethal Systemic Inflammation in a Murine Model of Rickettsia typhi Infection: Role of Neutrophils, Macrophages and NK Cells
Stefanie Papp et al. PLoS Negl Trop Dis. 2016.
Abstract
Rickettsia (R.) typhi is the causative agent of endemic typhus, an emerging febrile disease that is associated with complications such as pneumonia, encephalitis and liver dysfunction. To elucidate how innate immune mechanisms contribute to defense and pathology we here analyzed R. typhi infection of CB17 SCID mice that are congenic to BALB/c mice but lack adaptive immunity. CB17 SCID mice succumbed to R. typhi infection within 21 days and showed high bacterial load in spleen, brain, lung, and liver. Most evident pathological changes in R. typhi-infected CB17 SCID mice were massive liver necrosis and splenomegaly due to the disproportionate accumulation of neutrophils and macrophages (MΦ). Both neutrophils and MΦ infiltrated the liver and harbored R. typhi. Both cell populations expressed iNOS and produced reactive oxygen species (ROS) and, thus, exhibited an inflammatory and bactericidal phenotype. Surprisingly, depletion of neutrophils completely prevented liver necrosis but neither altered bacterial load nor protected CB17 SCID mice from death. Furthermore, the absence of neutrophils had no impact on the overwhelming systemic inflammatory response in these mice. This response was predominantly driven by activated MΦ and NK cells both of which expressed IFNγ and is considered as the reason of death. Finally, we observed that iNOS expression by MΦ and neutrophils did not correlate with R. typhi uptake in vivo. Moreover, we demonstrate that MΦ hardly respond to R. typhi in vitro. These findings indicate that R. typhi enters MΦ and also neutrophils unrecognized and that activation of these cells is mediated by other mechanisms in the context of tissue damage in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
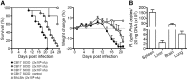
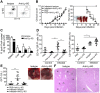
Fig 1. CB17 SCID mice succumb to R. typhi infection and develop systemic infection.
CB17 SCID mice were infected s.c. with 2×106, 2×104 or 2×102 sfu R. typhi into the base of the tail (n = 10 for each group). BALB/c mice received 2×106 sfu R. typhi via the same route (n = 11). Control CB17 SCID mice received PBS (n = 10). Survival rates (left; y-axis) and weight loss were monitored (right; y-axis) (A). Bacterial burden (y-axis) was determined in liver, spleen, brain and lung of CB17 SCID after s.c. infection with 2×106 sfu R. typhi (n = 10–11) by PrsA qPCR at the time of death (B). Data represent combined results from two independent experiments and show the mean ± SEM.
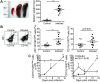
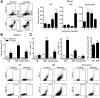
Fig 2. CB17 SCID mice develop splenomegaly which is largely due to the accumulation of MΦ and neutrophils.
CB17 SCID mice were infected s.c. with 2×106 sfu into the base of the tail. Control CB17 SCID mice received PBS. The photograph shows a representative spleen of a control mouse (left) and two R. _typhi_-infected CB17 SCID mice at the time of death (middle and right). Spleen weight (y-axis) was determined. The graph shows combined results from two independent experiments. Each dot represents a single mouse (n = 10–11). The mean ± SEM is presented. Data were analyzed with unpaired t test. Asterisks indicate statistically significant differences (***p<0.001) (A). The percentage of CD11b+GR1low MΦ/monocytes and CD11b+GR1hi neutrophils among spleen cells (y-axis) in CB17 SCID control animals (open circles) and R. _typhi_-infected CB17 SCID mice (black circles) was assessed at the time of death of the animals by flow cytometry. A representative staining for CD11b and GR1 and gating of cells is shown for a control mouse and a R. _typhi_-infected CB17 SCID mouse. Numbers indicate the percentage of the CD11b+GR1hi neutrophils and CD11b+GR1low MΦ/monocytes. Graphs show combined results from two independent experiments. Each dot in the graphs represents a single mouse (n = 10–11). The mean ± SEM is depicted. Statistical analysis was performed with student`s t-test after D´Agostino and Pearson normality test. Asterisks indicate statistically significant differences (** p<0.01) (B). BALB/c (n = 5–7) and CB17 SCID mice (n = 5–7) were infected s.c. with 2×106 sfu into the base of the tail. Total numbers of CD11b+GR1low MΦ/monocytes and CD11b+GR1hi neutrophils among spleen cells (y-axis) were assessed by flow cytometric staining as described above during the course of infection. Graphs show the total numbers of CD11b+GR1low MΦ/monocytes and CD11b+GR1hi neutrophils (y-axis) at the indicated point in time (x-axis). The mean ± SEM is depicted. Statistical analysis was performed with Kruskal-Wallis test followed by Dunn´s post-test comparing samples from infected mice with samples from control animals (day 0) (C).
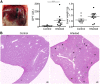
Fig 3. R. _typhi_-infected CB17 SCID mice develop severe liver necrosis.
CB17 SCID mice were infected s.c. with 2×106 sfu into the tail base while control mice received PBS. The photograph shows a representative liver of a R. _typhi_-infected CB17 SCID mouse at the time of death (left). GPT was measured in sera (y-axis) of control mice (open symbols) and R. _typhi_-infected CB17 SCID mice (black symbols) (middle) and liver weight (y-axis) was determined at the time of death (right). The graphs show combined results from two independent experiments (n = 10). Each dot represents a single mouse. The mean ± SEM is given. Statistical significance was analyzed by Mann Whitney U test after D´Agostino and Pearson normality test (A). Representative histological stainings of the liver from a control mouse (left) and a R. _typhi_-infected CB17 SCID mouse (right) with HE are depicted. Images were taken at 2-fold magnification. Black arrows point to necrotic lesions. White arrows indicate foci of infiltrating cells (B).
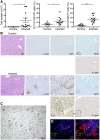
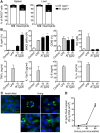
Fig 4. CD11b+GR1low MΦ/monocytes as well as of CD11b+GR1hi neutrophils infiltrate the liver.
CB17 SCID mice were infected s.c. with 2×106 sfu into the base of the tail. Control mice received PBS. At the time of death of R. _typhi_-infected mice the numbers of cellular isolates from liver (left) and the percentage of CD11b+GR1low MΦ/monocytes (middle) and CD11b+GR1hi neutrophils (right) (y-axis) were determined by flow cytometry. Graphs show combined results from two independent experiments and each dot represents a single mouse (n = 10–11). The mean ± SEM is depicted. Data were analyzed by Student`s t-test (left and right) or Mann Whitney test (middle) after D´Agostino and Pearson normality test. Asterisks indicate statistically significant differences (*p<0.05, *** p<0.001) (A). Serial sections of the liver from a control mouse and a R. _typhi_-infected CB17 SCID mouse were stained for IBA1, Ly-6G, iNOS and R. typhi as indicated. Pictures show a necrotic area. (B). In addition, several foci of infiltrating IBA1+ MΦ from the periphery were observed (C). Immunofluorescent co-stainings were performed for R. typhi (green) and Ly-6G or IBA1 (red) from the liver of an infected CB17 SCID mouse. Nuclei were stained with DAPI (blue) (D).
Fig 5. Neutrophils and MΦ release ROS and express iNOS.
CB17 SCID mice were infected s.c. with 2×106 sfu into the base of the tail. At the time of death blood, spleen and liver cell samples were analyzed by flow cytometry for ROS release, R. typhi uptake and iNOS expression. A representative dot plot of ROS staining in CD11b+Ly-6C+/-Ly-6G- MΦ/monocytes (upper panel) and CD11b+Ly-6G+ neutrophils (lower panel) from a control mouse (left) and a R. _typhi_-infected mouse (right) is shown. The graph depicts the mean ± SEM of the percentage of ROS-releasing cells (y-axis) among MΦ/monocytes and neutrophils (x-axis) determined in blood samples from two independent experiments (n = 10–11) (below) (A). Representative dot plots of spleen (B) and liver cells (C) gated on CD11b+ cells and further stained for GR1, intracellular R. typhi (upper panel) and iNOS (lower panel) are shown. Graphs show the percentage of R. typhi+ and iNOS+ cells (y-axis) among CD11b+GR1low MΦ/monocytes and CD11b+GR1hi neutrophils (x-axis). Data show the mean ± SEM of combined results from two independent experiments (n = 10–11). Statistical analysis was performed with Mann-Whitney U test (*p<0.05, **p<0.01, ***p<0.001) (B,C).
Fig 6. Neutrophil depletion completely prevents liver damage in CB17 SCID mice but does not protect from death.
CB17 SICD mice were infected s.c. with 2×106 sfu into the base of the tail. Neutrophils were depleted by intraperitoneal application of anti-Ly-6G antibody. Control animals received rat IgG2a isotype antibody. Depletion was first performed on day 6 post infection and then repeated every 3 days. Efficacy of depletion was assessed by flow cytometric staining of blood cells 1 day after the second depletion (day 10 post infection). A representative dot plot of blood cells from an isotype antibody-treated mouse (left) and a neutrophil-depleted mouse (right) stained for Ly-6G and CD11b is depicted (A). The health status of the mice was assessed over time (x-axis) using a clinical score (y-axis). Four groups of mice were analyzed (non-infected mice that were treated with PBS only (open circles) or PBS and anti-Ly-6G (black circles) and R. _typhi_-infected mice that were treated with isotype (open squares) or anti-Ly-6G antibody (black squares); n = 9–10 for each group). The data show the mean ± SEM of the clinical score obtained from two independent experiments. Survival rates (y-axis) of anti-Ly-6G- and isotype antibody-treated mice were compared using the Log-rank Mantel Cox test (p = 0.7778; non-significant). Spleens of R. _typhi_-infected neutrophil-depleted mice were still strongly enlarged. The inserted photograph shows two spleens from non-infected control mice that received PBS (left) and three spleens from neutrophil-depleted R. _typhi_-infected mice (B). Bacterial burden was determined by PrsA qPCR (y-axis) in liver, spleen, brain and lung (x-axis) of R. _typhi_-infected isotype-treated (white bars) or anti-Ly-6G-treated CB17 SCID mice (black bars) at the time of death (n = 10). The mean ± SEM is depicted (C). The percentage of CD11b+GR1hi neutrophils (left) and CD11b+GR1low MΦ/monocytes (right) (y-axis) in the spleens from the very same mice were determined by flow cytometry. Each dot represents a single mouse. The mean ± SEM is depicted. Data were analyzed by one-way ANOVA followed by Tukey post-test (left) or Kruskal Wallis test followed by Dunn´s post-test (right) after D´Agostino and Pearson normality test. Asterisks indicate statistically significant differences (*p<0.05, **p<0.01, ***p<0.001) (D). GPT was determined in sera of infected CB17 SCID mice that received isotype (open squares) or anti-Ly-6G antibody (black squares) (n = 8–9) at the time of death. Control animals were treated with anti-Ly-6G but received PBS instead of R. typhi (black circles). Each dot represents a single mouse. Mean ± SEM from combined results of two independent experiments are shown. Data were analyzed by Kruskal-Wallis test followed by Dunn´s post-test after D´Agostino and Pearson normality test. Asterisks indicate statistically significant differences (**p<0.01). Photographs show a representative liver of an isotype-treated (left) and an anti-Ly6G-treated R. _typhi_-infected CB17 SCID mouse (right) (E). Representative HE stainings of histological sections of the liver of an isotype-treated and an anti-Ly-6G-treated R. _typhi_-infected CB17 SCID mouse at the time of death are shown. Black arrows point to necrotic lesions. Necrosis was not observed in anti-Ly-6G-treated mice. Open arrows point to cellular infiltrates (F).
Fig 7. Neutrophil depletion does not alter systemic inflammatory response.
Plasma cytokine levels (y-axis) were measured at indicated points in time (x-axis) in R. _typhi_-infected BALB/c (open symbols, n = 5–8) and CB17 SCID mice (black symbols, n = 5–8) by bead-based LEGENDplex assay. Data represent combined results from two independent experiments and are shown as mean ± SEM. Statistical significance was determined using Kruskall-Wallis test followed by Dunn´s post-test comparing samples from infected mice with samples from control mice (day 0) (A). Plasma cytokine levels (y-axis) from R. _typhi_-infected and PBS-treated CB17 SCID mice that either received isotype antibody or anti-Ly-6G as indicated on the x-axis were measured by bead-based LEGENDplex assay at the time of death. Combined results from two independent experiments are shown (n = 8–9). Each dot represents a single mouse. The mean ± SEM is shown. Statistical analysis was performed with Kruskall-Wallis test followed by Dunn´s post-test (B). Asterisks indicate statistically significant differences (*p<0.05, **p<0.01, ***p<0.001).
Fig 8. Expansion of NK cells, MΦ and neutrophils and cytokine expression by these cell populations.
CB17 SCID mice (n = 8) were infected s.c. with 2×106 sfu into the base of the tail while control animals received PBS (n = 8). Blood cells were analyzed by flow cytometric staining of NKp46, CD11b and GR1. Cells were gated on NKp46+ (NK cells) and NKp46- cells that were further differentiated into MΦ (CD11b+GR1low) and neutrophils (CD11b+GR1hi) as indicated in the example dot plots on the left. Absolute numbers of NK cells, MΦ and neutrophils were determined per μl blood (y-axis) at indicated points in time (x-axis). Measurements from non-infected PBS-treated mice were used as "day 0" control. Statistical analysis was performed with Kruskall-Wallis test followed by Dunn´s post-test (A). 12h before the experiment was terminated (day 12) the animals received brefeldin A to prevent cytokine secretion by the cells in vivo. Spleen cells were stained for NKp46, CD11b, GR1, IFNγ and TNFα and analyzed by flow cytometry. Cells were gated as described above. Absolute cell counts (y-axis) of NK cells, MΦ and neutrophils as indicated on the x-axis were determined. Statistical analysis was performed with Mann-Whitney U test (two-tailed) (B). The percentage of IFNγ- and TNFα-expressing cells among NK cells, MΦ and neutrophils in the spleen of PBS-treated control mice (n = 7) and R. _typhi_-infected animals (n = 8) was analyzed. The dot plots below show example stainings of the indicated cell population. Graphs on the left and in the middle show the statistical analysis of the percentage of IFNγ-expressing and TNFα-expressing cells (y-axis) among each cell population (x-axis). The graph on the right shows the analysis of the absolute cell count of IFNγ-expressing cells (y-axis) among NK cells and MΦ (x-axis). Statistical analysis was performed with Mann-Whitney U test (two-tailed) (C). Asterisks indicate statistically significant differences (*p<0.05, **p<0.01, ***p<0.001).
Fig 9. MΦ hardly respond to R. typhi and are uncapable to kill the bacteria.
To analyze a correlation of bacterial uptake and activation status of CD11b+GR1low MΦ /monocytes and CD11b+GR1hi neutrophils in vivo the respective cell population in the spleen and liver of the mice described in Fig 5 was first gated on iNOS+ cells and then analyzed for bacterial content. The graphs show the percentage of R. _typhi_-positive cells (R. typhi+, black bars) and R. _typhi_-negative cells (R. typhi-, white bars) among the iNOS-expressing cells (y-axis) of the indicated cell population (x-axis). Statistical analysis was performed with Student´s T test (***p<0.001) (A). bmMΦ were infected with indicated amounts of purified bacteria per cell. Control cells were either left untreated (control) or stimulated with 100 ng/ml LPS (x-axis). After 24h cells were analyzed for the presence of R. typhi by flow cytometric staining. The percentage of R. typhi+ cells is shown (y-axis). MHCI and CD80 expression and the release of NO, TNFα, IL-12p70, IL-6 and IL-1 were analyzed after 48h (y-axis). Graphs show combined results from four independent experiments. Data were analyzed by one-way ANOVA followed by Tukey´s post-test. Asterisks indicate statistically significant differences compared to untreated cells (*p<0.05, **p<0.01) (B). bmMΦ were plated in chamber slides and either treated with heat-inactivated or living R. typhi as indicated. 10 copies per cell were used. After 48 h cells were stained for R. typhi with anti-R. typhi (BNI52) (green). Nuclei were stained with DAPI (blue). Images were taken at 800x magnification. One view of bmMΦ containing heat-inactivated, degrading bacteria and four views of bmMΦ containing living R. typhi are shown (C). Growth of R. typhi in bmMΦ that were infected with 5 copies per cell was analyzed. Bacterial content (y-axis) was quantified by PrsA qPCR at indicated points in time (x-axis). The graph shows combined results from three independent experiments (D).
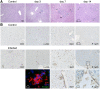
Fig 10. Temporary liver damage in BALB/c wild-type mice upon R. typhi infection.
BALB/c mice were infected s.c. with 2×106 sfu R. typhi. Liver sections were analyzed at indicated points in time post infection by staining with HE. Pictures show representative stainings of one out of five mice (A). Serial sections of the liver from PBS-treated BALB/c control mice or BALB/c mice infected s.c. with R. typhi as described above were stained for IBA1, Ly-6G, iNOS and R. typhi employing serum from a patient. For the staining of IBA1 two views are shown. In addition, co-staining of R. typhi (green), IBA1 (red) and DAPI (blue) was performed (B).
Similar articles
- GFPuv-Expressing Recombinant Rickettsia typhi: a Useful Tool for the Study of Pathogenesis and CD8+ T Cell Immunology in R. typhi Infection.
Hauptmann M, Burkhardt N, Munderloh U, Kuehl S, Richardt U, Krasemann S, Hartmann K, Krech T, Fleischer B, Keller C, Osterloh A. Hauptmann M, et al. Infect Immun. 2017 May 23;85(6):e00156-17. doi: 10.1128/IAI.00156-17. Print 2017 Jun. Infect Immun. 2017. PMID: 28289147 Free PMC article. - Cytotoxic effector functions of T cells are not required for protective immunity against fatal Rickettsia typhi infection in a murine model of infection: Role of TH1 and TH17 cytokines in protection and pathology.
Moderzynski K, Heine L, Rauch J, Papp S, Kuehl S, Richardt U, Fleischer B, Osterloh A. Moderzynski K, et al. PLoS Negl Trop Dis. 2017 Feb 21;11(2):e0005404. doi: 10.1371/journal.pntd.0005404. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28222146 Free PMC article. - Persisting Rickettsia typhi Causes Fatal Central Nervous System Inflammation.
Osterloh A, Papp S, Moderzynski K, Kuehl S, Richardt U, Fleischer B. Osterloh A, et al. Infect Immun. 2016 Apr 22;84(5):1615-1632. doi: 10.1128/IAI.00034-16. Print 2016 May. Infect Immun. 2016. PMID: 26975992 Free PMC article. - Macrophages make me sick: how macrophage activation states influence sickness behavior.
Moon ML, McNeil LK, Freund GG. Moon ML, et al. Psychoneuroendocrinology. 2011 Nov;36(10):1431-40. doi: 10.1016/j.psyneuen.2011.07.002. Epub 2011 Aug 19. Psychoneuroendocrinology. 2011. PMID: 21855222 Free PMC article. Review. - The Impact of Liver Failure on the Immune System.
Dąbrowska A, Wilczyński B, Mastalerz J, Kucharczyk J, Kulbacka J, Szewczyk A, Rembiałkowska N. Dąbrowska A, et al. Int J Mol Sci. 2024 Sep 1;25(17):9522. doi: 10.3390/ijms25179522. Int J Mol Sci. 2024. PMID: 39273468 Free PMC article. Review.
Cited by
- Comparative evaluation of two Rickettsia typhi-specific quantitative real-time PCRs for research and diagnostic purposes.
Papp S, Rauch J, Kuehl S, Richardt U, Keller C, Osterloh A. Papp S, et al. Med Microbiol Immunol. 2017 Feb;206(1):41-51. doi: 10.1007/s00430-016-0480-z. Epub 2016 Oct 1. Med Microbiol Immunol. 2017. PMID: 27696011 - Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts.
Torina A, Villari S, Blanda V, Vullo S, La Manna MP, Shekarkar Azgomi M, Di Liberto D, de la Fuente J, Sireci G. Torina A, et al. Int J Mol Sci. 2020 Jul 30;21(15):5437. doi: 10.3390/ijms21155437. Int J Mol Sci. 2020. PMID: 32751625 Free PMC article. Review. - Global Transcriptomic Profiling of Pulmonary Gene Expression in an Experimental Murine Model of Rickettsia conorii Infection.
Narra HP, Sahni A, Khanipov K, Fofanov Y, Sahni SK. Narra HP, et al. Genes (Basel). 2019 Mar 8;10(3):204. doi: 10.3390/genes10030204. Genes (Basel). 2019. PMID: 30857242 Free PMC article. - Serum cytokine responses in Rickettsia felis infected febrile children, Ghana.
Rauch J, Sothmann P, Aldrich C, Hogan B, Owusu-Dabo E, May J, Eibach D, Tappe D. Rauch J, et al. Med Microbiol Immunol. 2018 Aug;207(3-4):243-248. doi: 10.1007/s00430-018-0544-3. Epub 2018 May 8. Med Microbiol Immunol. 2018. PMID: 29736763 Free PMC article. - Rickettsia typhi and Haemophagocytic Syndrome.
Iaria C, Colomba C, Di Carlo P, Scarlata F, Tolomeo M, Cascio A. Iaria C, et al. Am J Trop Med Hyg. 2017 Nov;97(5):1632. doi: 10.4269/ajtmh.17-0606. Am J Trop Med Hyg. 2017. PMID: 29140237 Free PMC article. No abstract available.
References
- Sekeyova Z, Roux V, Raoult D. Phylogeny of Rickettsia spp. inferred by comparing sequences of 'gene D', which encodes an intracytoplasmic protein. Int J Syst Evol Microbiol. 2001;51(Pt 4):1353–60. Epub 2001/08/09. . - PubMed
- Rathi N, Rathi A. Rickettsial infections: Indian perspective. Indian Pediatr. 2010;47(2):157–64. Epub 2010/03/17. . - PubMed
MeSH terms
Substances
Grants and funding
The authors received no specific funding for this work.
LinkOut - more resources
Full Text Sources
Other Literature Sources