Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion - PubMed (original) (raw)
Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion
Lee Dolat et al. J Cell Biol. 2016.
Abstract
Macropinocytosis, the internalization of extracellular fluid and material by plasma membrane ruffles, is critical for antigen presentation, cell metabolism, and signaling. Macropinosomes mature through homotypic and heterotypic fusion with endosomes and ultimately merge with lysosomes. The molecular underpinnings of this clathrin-independent endocytic pathway are largely unknown. Here, we show that the filamentous septin GTPases associate preferentially with maturing macropinosomes in a phosphatidylinositol 3,5-bisphosphate-dependent manner and localize to their contact/fusion sites with macropinosomes/endosomes. Septin knockdown results in large clusters of docked macropinosomes, which persist longer and exhibit fewer fusion events. Septin depletion and overexpression down-regulates and enhances, respectively, the delivery of fluid-phase cargo to lysosomes, without affecting Rab5 and Rab7 recruitment to macropinosomes/endosomes. In vitro reconstitution assays show that fusion of macropinosomes/endosomes is abrogated by septin immunodepletion and function-blocking antibodies and is induced by recombinant septins in the absence of cytosol and polymerized actin. Thus, septins regulate fluid-phase cargo traffic to lysosomes by promoting macropinosome maturation and fusion with endosomes/lysosomes.
© 2016 Dolat and Spiliotis.
Figures
Figure 1.
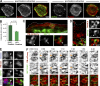
Septins localize to macropinosomes and their contact/fusion sites. (A) Confocal images of MDCKs stained for SEPT2 and F-actin with or without treatment with 0.1% Triton X-100 before fixation. (B) Bar graph shows the sum intensity (mean ± SEM) of SEPT2 per cell area (n = 15). AU, arbitrary units. (C and D) 3D-rendered confocal microscopy (C) and SIM (D) images of MDCK-PM-mCherry cells stained for endogenous SEPT2. Dashed lines outline the cell edge, and arrows point to SEPT2 at the contact sites of macropinocytic vacuoles. (E) MDCK-PM-mCherry cells were incubated with FITC-dextran and stained for SEPT2. 3D-rendered confocal images show SEPT2 at the periphery and in between (arrow) macropinosomes/endosomes. (F) MDCK-PM-mCherry cells were transfected with SEPT2-GFP and imaged live with wide-field deconvolution microscopy. Still frames show SEPT2 accumulation (arrows) at the site of fusion between two macropinosomes.
Figure 2.
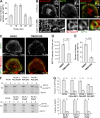
Septins associate preferentially with maturing macropinosomes in a PI(3,5)P2-dependent manner. (A) MDCKs were pulsed with TR-dextran for 5 min, chased for the indicated times, stained for SEPT2, and imaged with confocal microscopy. Bar graph shows the percentage (mean ± SEM) of dextran-containing macropinosomes/endosomes with SEPT2 (n = 12 cells). (B) Maximum-intensity projections of confocal stacks and SIM images of MDCKs transfected with ML1Nx2-GFP and stained for SEPT2. Insets show a vacuole (arrow) in higher magnification. (C) Maximum-intensity projections of confocal images of MDCK-PM-mCherry cells stained for SEPT2 after 2-h treatment with DMSO or YM201636 (800 nM). (D) Bar graph shows the sum intensity (mean ± SEM) of peripheral SEPT2 per square micrometer (n = 20 cells). AU, arbitrary units. (E) Bar graph shows the fraction (mean ± SEM) of TR-dextran puncta with SEPT2 (n = 20 cells) in MDCKs incubated with TR-dextran for 10 min after DMSO or YM201636 treatment. (F) Recombinant SEPT2/6/7 was mixed with liposomes of the indicated phosphoinositides and centrifuged on a sucrose gradient. Coomassie-stained gels show equal volumes from the top (liposome-bound SEPT2/6/7; B) and bottom (unbound SEPT2/6/7; U) fractions. (G) Bar graphs show the fraction of bound and unbound SEPT2/6/7 from three independent experiments. Error bars represent the maximum and minimum values from the three independent experiments.
Figure 3.
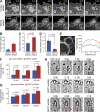
Septin depletion decreases macropinocytic fusion events and hinders macropinosome maturation and turnover. (A) Time-lapse frames from wide-field deconvolution microscopy show the dynamics of PM-mCherry–labeled macropinosomes in MDCK cells treated with control and SEPT2 shRNAs. Yellow arrow points to a cluster of macropinosomes that fuse into a single vacuole. (B) Control (n = 41) and SEPT2-depleted MDCK-PM-mCherry cells (n = 31) were imaged live every 5 s for 8 min. Bar graph shows percentage cells with a cluster of three or more macropinosomes formed from a lamellar ruffle. Error bars indicate the maximum and minimum values from three independent experiments. (C and D) Bar graphs show the lifetime (C) and fusion events (D) of nascent macropinosomes (n = 24–27). Error bars represent mean ± SEM. (E) Quantification of PM-mCherry fluorescence along the contacting (1) and noncontacting (2 and 3) segments of two macropinosomes from a SEPT2-depleted cell. AU, arbitrary units. (F and G) MDCKs were incubated with FITC-dextran for the indicated times. Bar graphshow the number of FITC-dextran–containing macropinosomes/endosomes (F) and their size (G) per cell (n = 18). Error bars represent mean ± SEM. (H) Still frames show the formation and detachment of PM-mCherry–labeled membrane tubules (arrows) in live MDCKs.
Figure 4.
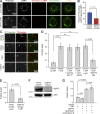
Septins regulate the lysosomal delivery of fluid-phase cargo. (A) MDCKs were transfected for 48 h with control or SEPT2 shRNAs and GFP or shRNA-resistant SEPT2-YFP (rescue). After a 5-min pulse/15-min chase with TR-dextran, cells were stained for LAMP1. Images show maximum-intensity projections of confocal image stacks. (B) Bar graph shows percentage (mean ± SEM) of TR-dextran particles that colocalize with LAMP1 (n = 20 cells). (C) Plot shows percentage (mean ± SEM) of TR-dextran with LAMP1 in control and SEPT2-depleted MDCKs (n = 15), which were pulsed with TR-dextran for 5 min and chased for the indicated times. (D) Lysates from MDCKs that stably express SEPT2-YFP at low and high levels were analyzed by SDS-PAGE and blotted for SEPT2. (E) Plot shows percentage (mean ± SEM) of TR-dextran with LAMP1 in MDCK cells (n = 14) that express SEPT2-YFP at low and high levels. Cells were pulsed with TR-dextran for 5 min and chased for the indicated times.
Figure 5.
Septins are required for fusion of macropinosomes/endosomes with lysosomes and promote fusion directly in an in vitro reconstitution assay. (A) MDCKs were treated with control or SEPT2 siRNA, and two rounds of 5-min pulse/15-min chase were performed with TR- and FITC-dextran, successively. Cells were stained with anti-LAMP1 and imaged with confocal microscopy. Images show maximum-intensity projections of TR-dextran and LAMP1 and their areas of overlap (pseudo-colored in red masks), as well as FITC-dextran (green) and its overlay with the pseudo TR-dextran/LAMP1 channel. (B) Bar graph shows percentage TR-dextran/LAMP1 that contains FITC-dextran (n = 18 cells). (C and D) MDCKs were separately incubated with TR- or FITC-labeled dextran for 5 min, and postnuclear supernatants were mixed with canine kidney cytosol without or with ATP (3.3 mM) plus cytochalasin D (10 µM), nocodazole (10 µM), and control or anti-SEPT2 IgG. Images (C) show dextran-containing macropinosomes/endosomes after incubation in the indicated conditions. Bar graph (D) shows the fraction (mean ± SEM) of fused macropinosomes/endosomes with both TR- and FITC-labeled dextran (n = 15 images). n.s., not significant. (E) Bar graph shows the fraction (mean ± SEM) of fused endosomes after incubation of PNS with ATP and whole or SEPT2 immunodepleted (ΔSEPT2) cytosol (n = 15). (F) Gel shows equal volumes of whole and ΔSEPT2 cytosol blotted for SEPT2 and actin. (G) Bar graphs show the fraction (mean ± SEM) of fused endosomes after incubation of postnuclear supernatants with ATP and SEPT2 or SEPT2/6/7 (2.5 µM) in the presence/absence of 10 µM cytochalasin D (n = 15).
Similar articles
- Macropinosome maturation and fusion with tubular lysosomes in macrophages.
Racoosin EL, Swanson JA. Racoosin EL, et al. J Cell Biol. 1993 Jun;121(5):1011-20. doi: 10.1083/jcb.121.5.1011. J Cell Biol. 1993. PMID: 8099075 Free PMC article. - The PripA-TbcrA complex-centered Rab GAP cascade facilitates macropinosome maturation in Dictyostelium.
Tu H, Wang Z, Yuan Y, Miao X, Li D, Guo H, Yang Y, Cai H. Tu H, et al. Nat Commun. 2022 Apr 4;13(1):1787. doi: 10.1038/s41467-022-29503-1. Nat Commun. 2022. PMID: 35379834 Free PMC article. - The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms.
Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, Zerial M. Schnatwinkel C, et al. PLoS Biol. 2004 Sep;2(9):E261. doi: 10.1371/journal.pbio.0020261. Epub 2004 Aug 24. PLoS Biol. 2004. PMID: 15328530 Free PMC article. - Class III phosphatidylinositol 3-kinase and its catalytic product PtdIns3P in regulation of endocytic membrane traffic.
Raiborg C, Schink KO, Stenmark H. Raiborg C, et al. FEBS J. 2013 Jun;280(12):2730-42. doi: 10.1111/febs.12116. Epub 2013 Feb 6. FEBS J. 2013. PMID: 23289851 Review. - Membrane traffic to and from lysosomes.
Luzio JP, Pryor PR, Gray SR, Gratian MJ, Piper RC, Bright NA. Luzio JP, et al. Biochem Soc Symp. 2005;(72):77-86. doi: 10.1042/bss0720077. Biochem Soc Symp. 2005. PMID: 15649132 Review.
Cited by
- The Role and Therapeutic Potential of Macropinocytosis in Cancer.
Qiu Z, Liu W, Zhu Q, Ke K, Zhu Q, Jin W, Yu S, Yang Z, Li L, Sun X, Ren S, Liu Y, Zhu Z, Zeng J, Huang X, Huang Y, Wei L, Ma M, Lu J, Chen X, Mou Y, Xie T, Sui X. Qiu Z, et al. Front Pharmacol. 2022 Aug 15;13:919819. doi: 10.3389/fphar.2022.919819. eCollection 2022. Front Pharmacol. 2022. PMID: 36046825 Free PMC article. Review. - Septins, a cytoskeletal protein family, with emerging role in striated muscle.
Gönczi M, Dienes B, Dobrosi N, Fodor J, Balogh N, Oláh T, Csernoch L. Gönczi M, et al. J Muscle Res Cell Motil. 2021 Jun;42(2):251-265. doi: 10.1007/s10974-020-09573-8. Epub 2020 Jan 18. J Muscle Res Cell Motil. 2021. PMID: 31955380 Free PMC article. Review. - From Pinocytosis to Methuosis-Fluid Consumption as a Risk Factor for Cell Death.
Ritter M, Bresgen N, Kerschbaum HH. Ritter M, et al. Front Cell Dev Biol. 2021 Jun 23;9:651982. doi: 10.3389/fcell.2021.651982. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249909 Free PMC article. Review. - Macropinocytosis: A Metabolic Adaptation to Nutrient Stress in Cancer.
Recouvreux MV, Commisso C. Recouvreux MV, et al. Front Endocrinol (Lausanne). 2017 Sep 29;8:261. doi: 10.3389/fendo.2017.00261. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29085336 Free PMC article. Review. - Lysosomal positioning diseases: beyond substrate storage.
Scerra G, De Pasquale V, Scarcella M, Caporaso MG, Pavone LM, D'Agostino M. Scerra G, et al. Open Biol. 2022 Oct;12(10):220155. doi: 10.1098/rsob.220155. Epub 2022 Oct 26. Open Biol. 2022. PMID: 36285443 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases