Development of a Quantitative BRET Affinity Assay for Nucleic Acid-Protein Interactions - PubMed (original) (raw)
Development of a Quantitative BRET Affinity Assay for Nucleic Acid-Protein Interactions
Timothy A Vickers et al. PLoS One. 2016.
Abstract
Protein-nucleic acid interactions play a crucial role in the regulation of diverse biological processes. Elucidating the roles that protein-nucleic acid complexes play in the regulation of transcription, translation, DNA replication, repair and recombination, and RNA processing continues to be a crucial aspect of understanding of cell biology and the mechanisms of disease. In addition, proteins have been demonstrated to interact with antisense oligonucleotide therapeutics in a sequence and chemistry dependent manner, influencing ASO potency and distribution in cells and in vivo. While many assays have been developed to measure protein-nucleic acid interactions, many suffer from lack of throughput and sensitivity, or challenges with protein purification and scalability. In this report we present a new BRET assay for the analysis of DNA-protein interactions which makes use of an extremely bright luciferase as a tag for the binding protein, along with a long-wavelength fluorophore conjugated to the nucleic acid. The resulting assay is high throughput, sensitive, does not require protein purification, and even allows for quantitative characterization of these interactions within the biologically relevant context of whole cells.
Conflict of interest statement
This study was funded by Ionis Pharmaceuticals, Inc. The funder provided support in the form of salaries for authors TAV and STC but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
Fig 1
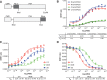
A) General cloning strategy for NLuc fusion proteins. P54nrb cDNA was amplified by PCR to include an N-terminal XhoI site and a C-terminal EcoRI site. The resulting cDNA was cloned in frame using the same sites in the plasmid pFN31K Nluc. For cloning into pFC32K Nluc the cDNA was amplified by PCR to include an N-terminal NheI site and a C-terminal XhoI site. Primer sequences can be found in S2 Table. Expression of all clones is driven from the CMV promoter. The resulting fusion plasmids were expressed in HeLa cells. B) ASO/BRET affinity for p54nrb. P54nrb/NLuc fusion proteins were immunopurified as detailed in Materials and Methods and subsequently incubated with Alexa 594 conjugated 5-10-5 cEt gap-mer ASO at concentrations ranging from 10 pM to 10 μM. BRET ratios were determined for P54nrb-NLuc (green, black) or NLuc-P54nrb (red, blue) with either a 3’ conjugated (766636) or 5’ conjugated (766635) ASO. Concentration response curves and KD’s (nM) for two independent experiments are shown. C) ASO/BRET binding affinity varies with chemistry of the 2’ modification. ASO/BRET assay was performed with P54nrb-NLuc fusion and 5’ conjugated 5-10-5 ASOs at concentrations ranging from 10 pM to 10 μM. 2’-F, red; cEt, blue; MOE, green. D) Relative affinities for 2’F (red), cET (blue), and MOE (green) gap-mer ASO as determined by competitive ASO binding to the NLuc/P54nrb fusion protein in the BRET assay. 10 nM 3’ Alexa conjugated cEt ASO (766636) was competed with unconjugated 5–10–5 2′-F (red), MOE (green), or cEt (blue) gap-mer ASO at concentrations from 0.1 to 1000 nM. Relative KD’s are shown. Data in panels C and D are mean ± SEM from 3–4 independent experiments.
Fig 2. ASO/BRET binding affinities for various NLuc fusion proteins.
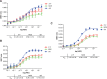
NLuc protein fusions were constructed, expressed, and immunopurified as detailed in Materials and Methods. Binding affinities were determined by incubating 106 RLU of immunopurified fusion protein with a 3’ ALEXA 594 conjugated 5-10-5 2’F gap-mer ASO (766638) at concentrations from 1 pM to 1 μM. Data are plotted as the percent of the maximal BRET ratio to control for differences in BRET amplitude for the various proteins. KD’s were determined using GraphPad Prism software. Similar experiments were performed for cEt (766636) and MOE (766634) gap-mer ASOs. The KD’s can be found in Table 1.
Fig 3. ASO binding to RNase H1 is highly specific.
A) NLuc-RNAseH1 fusion (red) was constructed as described in Materials and Methods. The NLuc/Barnase fusion (blue) was produced from the plasmid pFN31K Nluc CMV-neo (Promega), whereas the NLuc only plasmid (green) was generated by deletion of the barnase coding region from the same plasmid. Proteins were expressed then immunopurified using and antibody specific for NLuc. Binding affinities were determined by incubating 106 RLU of immunopurified protein with a 3’ ALEXA 594 conjugated 5-10-5 2’F (766638, solid lines) or MOE gap-mer (766634, dashed lines) ASOs at concentrations from 0.1 nM to 1 μM. B) The RNAse H1 hybrid binding domain (HBD) was deleted from NLuc/RNAseH1 by SDM. NLuc/RNAseH1 (solid lines) and NLuc/RNAseH1-HBD (dashed line) were expressed then immunopurified as above. Binding affinities were determined by incubating 106 RLU of immunopurified protein with a 3’ ALEXA 594 conjugated PO ASO with or without complementary PO DNA or RNA. BRET rations were plotted and KD’s (nM) determined for single stranded DNA (D, red), DNA/DNA duplex (D/D, green), or DNA/RNA heteroduplex (D/R, blue). Data shown are mean ± SEM from 3 independent experiments.
Fig 4. ASOs interaction with protein domains is specific and chemistry dependent.
A) cEt gap-mer ASO binds to La RNA binding domains 1 and 2. Deletion mutants were generated from Nluc-La by SDM, then expressed and immunopurified using an antibody to La. Binding affinities were determined by incubating 106 RLU of immunopurified protein with a 3’ ALEXA 594 conjugated 5-10-5 cEt gap-mer ASO (766636) at concentrations ranging from 100 pM to 10 μM. Data are plotted as the percent of the maximal BRET ratio to control for differences in BRET amplitude for the full length La (red), ∆La motif (violet), ∆RRM1 (green), ∆RRM2 (blue), or ∆RRM1/2 (black). B) cEt gap-mer ASO interacts with P54nrb at RRM1 and RRM2. Deletion mutants were generated from P54nrb/Nluc by SDM, then expressed and immunopurified using an antibody to P54nrb (Millipore). Binding affinities were determined by incubating 106 RLU of immunopurified proteins with a 3’ ALEXA 594 conjugated 5-10-5 2’F gap-mer ASO (766638) at concentrations ranging from 100 pM to 1 μM. Concentration curves were plotted for BRET ratios using GraphPad PRISM software for full length P54nrb (red), ∆RRM1 (green), ∆RRM2 (blue), or ∆RRM1/2 (black). Data shown are mean ± SEM from 3–4 independent experiments.
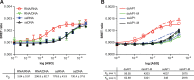
Fig 5. ASO binding affinity to P54nrb is highly sequence dependent.
A) Competitive ASO/BRET binding of 3-10-3 cEt ASOs to P54nrb. Binding affinities were determined by incubating 106 RLU of immunopurified NLuc/P54nrb fusion protein with 10 nM 3’ ALEXA 594 conjugated 5-10-5 2’F gap-mer ASO (766638) along with unconjugated TAAR5 3-10-3 cEt ASOs at concentrations ranging from 1 nM to 3 μM. Concentration curves are plotted for BRET ratios in the presence of each unconjugated ASO. TAAR5 ASO sequences and KD’s can be found in Table 2. B) P54nrb competitive fluorescent polarization assay. Competitive FP was performed with the same set of TAAR5 ASOs as detailed in Materials and Methods. C) Correlation between KD’s for ASO binding obtained by BRET and FP.
Fig 6. ASO/BRET in lysates and permeabilized cells.
LRPPRC/NLuc was constructed as detailed in Materials and Methods. The fusion protein was expressed by transient transfection in Hela cells. Cell lysate was prepared and the fusion protein immunopreciptated from ½ of the lysate with an antibody to NLuc. For ASO/BRET using the IP’ed protein (A) and cell lysate (B), binding affinities were determined by incubating 106 RLU of LRPPRC/NLuc fusion protein with 3’ ALEXA 594 conjugated 5-10-5 gap-mer ASO at concentrations between 3 pM to 300 nM. Concentration curves were plotted for BRET ratios using GraphPad PRISM software for 2’F (766638, red), MOE (766634, green), or cEt (766636 blue) gap-mer ASOs. For Nano BRET in intact cells (C), the HeLa cells expressing the LRPPRC/NLuc fusion were seeded in 96-well plates at 5000 cells/well. 24 hours after the initiation of transfection, cells were permeabilzed with 25 ng/mL digitonin in OptiMEM media plus 5-10-5 gap-mer ASO at concentrations between 3 pM to 300 nM. After a 15 minute incubation NanoGlo substrate was added and BRET ratios determined as above. Data shown are mean ± SEM from 3 independent experiments.
Fig 7. BRET with RNA and DNA binding proteins.
A) Staufen 1 specifically binds RNA duplexes. An NLuc/STAU1 fusion was constructed and expressed as detailed in Materials and Methods. The protein was immunoprecipitated from the cell lysate using an STAU1 antibody (Millipore AB5781), then binding affinities were determined by incubating 106 RLU of immunopurified fusion protein with a 3’ ALEXA 594 conjugated PO RNA 20-mer hybridized with a 40 nt complementary RNA strand (RNA/RNA; red) or a 3’ ALEXA 594 conjugated PO DNA 20-mer hybridized with the 40 nt complementary RNA strand (RNA/DNA; green) at concentrations ranging from 10 pM to 3 μM. Binding of the ssRNA (blue) and ssDNA (black) was also evaluated. Data is plotted as BRET ratio vs concentration RNA and represent mean ± SEM from 3 independent experiments. B) C-Jun binding to AP1 dsDNA by BRET. An amino-terminal NLuc fusion was constructed with the C-Jun protein (NLuc/cJun). The fusion protein was expressed by transient transfection in Hela cells which were subsequently seeded in 96 well plates at 5000 cells/well. 24 hours after the initiation of transfection, cells were permeabilzed with 25 ng/mL digitonin in OPtiMEM media plus PO DNA comprising the ds AP1 consensus sequence or a mutant AP1 site (AP1-M) at concentrations between 1 nM to 10 μM. Single stranded DNAs without the complementary DNA strand were also included in the BRET assay. After a 15 minute incubation NLuc substrate was added and BRET ratios determined as detailed above. Concentration response curves and KD’s (nM) for two independent experiments are shown.
Similar articles
- Origins of the Increased Affinity of Phosphorothioate-Modified Therapeutic Nucleic Acids for Proteins.
Hyjek-Składanowska M, Vickers TA, Napiórkowska A, Anderson BA, Tanowitz M, Crooke ST, Liang XH, Seth PP, Nowotny M. Hyjek-Składanowska M, et al. J Am Chem Soc. 2020 Apr 22;142(16):7456-7468. doi: 10.1021/jacs.9b13524. Epub 2020 Apr 10. J Am Chem Soc. 2020. PMID: 32202774 - MicroScale Thermophoresis: A Rapid and Precise Method to Quantify Protein-Nucleic Acid Interactions in Solution.
Mueller AM, Breitsprecher D, Duhr S, Baaske P, Schubert T, Längst G. Mueller AM, et al. Methods Mol Biol. 2017;1654:151-164. doi: 10.1007/978-1-4939-7231-9_10. Methods Mol Biol. 2017. PMID: 28986788 - Quantitative approaches to monitor protein-nucleic acid interactions using fluorescent probes.
Pagano JM, Clingman CC, Ryder SP. Pagano JM, et al. RNA. 2011 Jan;17(1):14-20. doi: 10.1261/rna.2428111. Epub 2010 Nov 22. RNA. 2011. PMID: 21098142 Free PMC article. Review. - Protein-nucleic acid complexes: Docking and binding affinity.
Gromiha MM, Harini K. Gromiha MM, et al. Curr Opin Struct Biol. 2025 Feb;90:102955. doi: 10.1016/j.sbi.2024.102955. Epub 2024 Nov 30. Curr Opin Struct Biol. 2025. PMID: 39616716 Review.
Cited by
- Nusinersen as a Therapeutic Agent for Spinal Muscular Atrophy.
Li Q. Li Q. Yonsei Med J. 2020 Apr;61(4):273-283. doi: 10.3349/ymj.2020.61.4.273. Yonsei Med J. 2020. PMID: 32233169 Free PMC article. Review. - Gymnotic Delivery of LNA Mixmers Targeting Viral SREs Induces HIV-1 mRNA Degradation.
Hillebrand F, Ostermann PN, Müller L, Degrandi D, Erkelenz S, Widera M, Pfeffer K, Schaal H. Hillebrand F, et al. Int J Mol Sci. 2019 Mar 3;20(5):1088. doi: 10.3390/ijms20051088. Int J Mol Sci. 2019. PMID: 30832397 Free PMC article. - Insights into innate immune activation via PS-ASO-protein-TLR9 interactions.
Pollak AJ, Zhao L, Vickers TA, Huggins IJ, Liang XH, Crooke ST. Pollak AJ, et al. Nucleic Acids Res. 2022 Aug 12;50(14):8107-8126. doi: 10.1093/nar/gkac618. Nucleic Acids Res. 2022. PMID: 35848907 Free PMC article. - Profiling the interactome of oligonucleotide drugs by proximity biotinylation.
Hanswillemenke A, Hofacker DT, Sorgenfrei M, Fruhner C, Franz-Wachtel M, Schwarzer D, Maček B, Stafforst T. Hanswillemenke A, et al. Nat Chem Biol. 2024 May;20(5):555-565. doi: 10.1038/s41589-023-01530-z. Epub 2024 Jan 17. Nat Chem Biol. 2024. PMID: 38233583 Free PMC article. - Towards next generation antisense oligonucleotides: mesylphosphoramidate modification improves therapeutic index and duration of effect of gapmer antisense oligonucleotides.
Anderson BA, Freestone GC, Low A, De-Hoyos CL, Iii WJD, Østergaard ME, Migawa MT, Fazio M, Wan WB, Berdeja A, Scandalis E, Burel SA, Vickers TA, Crooke ST, Swayze EE, Liang X, Seth PP. Anderson BA, et al. Nucleic Acids Res. 2021 Sep 20;49(16):9026-9041. doi: 10.1093/nar/gkab718. Nucleic Acids Res. 2021. PMID: 34417625 Free PMC article.
References
- Jayaram B, Jain T (2004) The Role of Water in Protein-DNA Recognition. Annual Review of Biophysics and Biomolecular Structure 33: 343–361. - PubMed
- Tolstorukov MY, Jernigan RL, Zhurkin VB (2004) Protein–DNA Hydrophobic Recognition in the Minor Groove is Facilitated by Sugar Switching. Journal of Molecular Biology 337: 65–76. - PubMed
- Rhodes D, Burley SK (2000) Protein–nucleic acid interactions: Editorial overview. Current Opinion in Structural Biology 10: 75–77. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources