Platelet Supernatant Suppresses LPS-Induced Nitric Oxide Production from Macrophages Accompanied by Inhibition of NF-κB Signaling and Increased Arginase-1 Expression - PubMed (original) (raw)
Platelet Supernatant Suppresses LPS-Induced Nitric Oxide Production from Macrophages Accompanied by Inhibition of NF-κB Signaling and Increased Arginase-1 Expression
Yusuke Ando et al. PLoS One. 2016.
Abstract
We previously reported that mouse bone marrow-derived macrophages (BMDMs) that had been co-cultured with platelets exhibited lower susceptibility to bacterial lipopolysaccharide (LPS) and produced lower levels of nitric oxide (NO) and inflammatory cytokines including TNF-α and IL-6. The suppression of macrophage responses was mediated, at least in part, by platelet supernatant. In the present study, we assessed phenotypic changes of BMDMs induced by incubation with the supernatant from thrombin-activated platelets (PLT-sup) and found that BMDMs cultured with PLT-sup (PLT-BMDMs) expressed a lower level of inducible NO synthase (iNOS) and a higher level of arginase-1, both of which are involved in the L-arginine metabolism, upon stimulation with LPS or zymosan. We also examined possible modulation of the NF-κB signaling pathway and observed suppression of IκBα phosphorylation and a decrease of NF-κB p65 expression in LPS-stimulated PLT-BMDMs. These results suggest that PLT-sup suppresses inflammatory responses of BMDMs via negative regulation of NF-κB signaling leading to lowered expression of iNOS and enhanced L-arginine catabolism by arginase-1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Fig 1. Attenuation of LPS-induced NO production from BMDMs by PLT-sup.
BMDMs (2.5 × 106 cells) were cultured for 24 h with PLT-sup (PLT-BMDMs) or 0.5 U/mL thrombin alone (control BMDMs) in a 3.5 cm dish, and stimulated with complete medium containing LPS (50 ng/mL) for 0–24 h. The production levels of NO2- in the culture supernatant were determined by Griess reaction using a calibration curve for NaNO2. Experiments were performed in quintuplicate and repeated four times. Data are presented as the mean ± SEM. *p < 0.05, **p < 0.01 vs. control BMDMs. Representative results of the four experiments are shown.
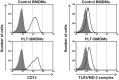
Fig 2. Cell surface expression of CD14 and the TLR4/MD-2 complex in PLT-BMDMs.
PLT-BMDMs and control BMDMs were incubated with anti-mouse CD16/32 at 4°C for 10 min, and then incubated with biotin anti-mouse CD14 antibody or rat anti-mouse TLR4/MD-2 complex antibody at 4°C for 30 min. After incubation with streptavidin-PE or anti-rat IgG-Alexa Fluor 647 antibody at 4°C for 30 min, cells were analyzed by FACSVerse (BD Biosciences). The data are shown as histograms representing the number of cells (Y-axis) against the log of fluorescence intensity (X-axis). The black lines represent cells with primary antibody, and the gray-shaded areas represent cells without primary antibody. Experiments were repeated three times, and representative results are shown.
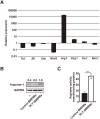
Fig 3. Induction of arginase-1 expression by PLT-sup in BMDMs.
(A) The gene expressions of Tnf, Il6, Il1b, Nos2, Arg1, Fizz1, Ym1, and Mrc1 in PLT-BMDMs and control BMDMs were analyzed by RT-qPCR with the relative standard curve method using Gapdh as an internal control. The gene expression in PLT-BMDMs was represented as the value relative to gene expression in control BMDMs. Experiments were performed in triplicate and repeated three times. Data are presented as the mean ± SEM, and representative results of the three experiments are shown. (B) PLT-BMDMs and control BMDMs were lysed in 1 × SDS sample buffer, and the resultant lysates were subjected to western blotting analysis with antibodies against arginase-1 or GAPDH. The relative intensity of each arginase-1 band after normalization to the corresponding levels of GAPDH is shown above each blot. Experiments were repeated five times, and representative results are shown. (C) PLT-BMDMs and control BMDMs (4 × 105 cells) were lysed with 0.1% Triton X-100 (100 μL) containing a protease inhibitor cocktail for 10 min at room temperature, and the lysate was assayed for arginase activity as described in the Materials and Methods. Experiments were performed in quintuplicate and repeated five times. Data are presented as the mean ± SEM. **p < 0.01 vs. control BMDMs. Representative results of the five experiments are shown.
Fig 4. Expression of iNOS and arginase-1 in BMDMs after LPS stimulation.
(A) PLT-BMDMs and control BMDMs (2.5 × 106 cells) were stimulated with LPS (50 ng/mL) for 24 h, and the gene expressions of Nos2 and Arg1 were analyzed by RT-qPCR with the relative standard curve method using Gapdh as an internal control. The gene expression is represented as the value relative to gene expression in the original BMDMs. Experiments were performed in quintuplicate and repeated four times. The data are presented as the mean ± SEM. ***p < 0.005 vs. control BMDMs. Representative results of the four experiments are shown. (B) PLT-BMDMs and control BMDMs (2.5 × 106 cells) were stimulated with LPS (50 ng/mL) for 0–24 h. Cells were then lysed in 1 × SDS sample buffer, and the cell lysates were subjected to western blotting analysis with antibodies against iNOS, arginase-1 or GAPDH. The relative intensity of each iNOS or arginase-1 band after normalization to the levels for GAPDH is shown in the lower panel. Experiments were repeated four times, and representative results are shown. (C) PLT-BMDMs and control BMDMs (2.5 × 106 cells) were stimulated with zymosan (25 or 100 μg/mL) for 12 h, and then cell lysates were subjected to western blotting analysis with antibodies against iNOS, arginase-1 or GAPDH. C, control BMDMs; P, PLT-BMDMs. The relative intensity of each iNOS or arginase-1 band after normalization to the levels for GAPDH is shown in the lower panel. Experiments were repeated four times, and representative results are shown.
Fig 5. Western blotting analyses of NF-κB and MAPK signaling pathways in BMDMs after LPS stimulation.
(A) PLT-BMDMs and control BMDMs (2.5 × 106 cells) were stimulated with LPS (50 ng/mL) for 0–120 min. Cells were then lysed in 1 × SDS sample buffer, and the cell lysates were subjected to western blotting analysis with antibodies against phospho-IκBα, total IκBα, phospho-NF-κB p65, total NF-κB p65, phospho-p38 MAPK, total p38 MAPK, phospho-JNK, total JNK, phospho-ERK1/2, total ERK1/2 or GAPDH. The relative intensity of each band after normalization to the corresponding level of GAPDH is shown in the right panel. Experiments were repeated three times, and representative results are shown. (B) PLT-BMDMs and control BMDMs (2.5 × 106 cells) that had been stimulated with LPS (50 ng/mL) for 0–120 min were separated into cytoplasmic and nuclear fractions. Each fraction was subjected to western blotting analysis with antibodies against NF-κB p65. GAPDH and histone H3 were used as controls for the cytoplasmic and nuclear fractions, respectively. The relative intensity of each band after normalization to the level of GAPDH or histone H3 is shown in the lower panel. Experiments were repeated three times, and representative results are shown.
Similar articles
- Platelets attenuate production of cytokines and nitric oxide by macrophages in response to bacterial endotoxin.
Ando Y, Oku T, Tsuji T. Ando Y, et al. Platelets. 2016 Jun;27(4):344-50. doi: 10.3109/09537104.2015.1103369. Epub 2015 Nov 20. Platelets. 2016. PMID: 26588084 - Suppression of inflammatory responses by handelin, a guaianolide dimer from Chrysanthemum boreale, via downregulation of NF-κB signaling and pro-inflammatory cytokine production.
Pyee Y, Chung HJ, Choi TJ, Park HJ, Hong JY, Kim JS, Kang SS, Lee SK. Pyee Y, et al. J Nat Prod. 2014 Apr 25;77(4):917-24. doi: 10.1021/np4009877. Epub 2014 Apr 1. J Nat Prod. 2014. PMID: 24689881 - Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages.
Kim JY, Park SJ, Yun KJ, Cho YW, Park HJ, Lee KT. Kim JY, et al. Eur J Pharmacol. 2008 Apr 14;584(1):175-84. doi: 10.1016/j.ejphar.2008.01.032. Epub 2008 Feb 5. Eur J Pharmacol. 2008. PMID: 18295200 - Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-κB and MAPK signaling pathway in LPS-induced RAW 264.7 macrophages.
Li D, Chen J, Ye J, Zhai X, Song J, Jiang C, Wang J, Zhang H, Jia X, Zhu F. Li D, et al. J Ethnopharmacol. 2017 Jan 20;196:66-74. doi: 10.1016/j.jep.2016.12.007. Epub 2016 Dec 15. J Ethnopharmacol. 2017. PMID: 27989509
Cited by
- Establishment of a Cell Suspension Culture of Ageratina pichinchensis (Kunth) for the Improved Production of Anti-Inflammatory Compounds.
Sánchez-Ramos M, Alvarez L, Romero-Estrada A, Bernabé-Antonio A, Marquina-Bahena S, Cruz-Sosa F. Sánchez-Ramos M, et al. Plants (Basel). 2020 Oct 21;9(10):1398. doi: 10.3390/plants9101398. Plants (Basel). 2020. PMID: 33096626 Free PMC article. - The In Vitro and In Vivo Anti-Inflammatory Effects of a Phthalimide PPAR-γ Agonist.
Su M, Cao J, Huang J, Liu S, Im DS, Yoo JW, Jung JH. Su M, et al. Mar Drugs. 2017 Jan 4;15(1):7. doi: 10.3390/md15010007. Mar Drugs. 2017. PMID: 28054961 Free PMC article. - Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro.
Nasirzade J, Kargarpour Z, Hasannia S, Strauss FJ, Gruber R. Nasirzade J, et al. J Periodontol. 2020 Feb;91(2):244-252. doi: 10.1002/JPER.19-0216. Epub 2019 Sep 14. J Periodontol. 2020. PMID: 31376159 Free PMC article. - Saffron extract interferes with lipopolysaccharide-induced brain activation of the kynurenine pathway and impairment of monoamine neurotransmission in mice.
Monchaux de Oliveira C, Morael J, Guille A, Amadieu C, Vancassel S, Gaudout D, Capuron L, Pourtau L, Castanon N. Monchaux de Oliveira C, et al. Front Nutr. 2023 Oct 5;10:1267839. doi: 10.3389/fnut.2023.1267839. eCollection 2023. Front Nutr. 2023. PMID: 37867499 Free PMC article. - Green Tea Polyphenol EGCG Attenuates MDSCs-mediated Immunosuppression through Canonical and Non-Canonical Pathways in a 4T1 Murine Breast Cancer Model.
Xu P, Yan F, Zhao Y, Chen X, Sun S, Wang Y, Ying L. Xu P, et al. Nutrients. 2020 Apr 10;12(4):1042. doi: 10.3390/nu12041042. Nutrients. 2020. PMID: 32290071 Free PMC article.
References
MeSH terms
Substances
Grants and funding
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan, by Japan Agency for Medical Research and Development (AMED), and by Hoshi University Otani Research Grants.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials