FHF-independent conduction of action potentials along the leak-resistant cerebellar granule cell axon - PubMed (original) (raw)
FHF-independent conduction of action potentials along the leak-resistant cerebellar granule cell axon
Katarzyna Dover et al. Nat Commun. 2016.
Abstract
Neurons in vertebrate central nervous systems initiate and conduct sodium action potentials in distinct subcellular compartments that differ architecturally and electrically. Here, we report several unanticipated passive and active properties of the cerebellar granule cell's unmyelinated axon. Whereas spike initiation at the axon initial segment relies on sodium channel (Nav)-associated fibroblast growth factor homologous factor (FHF) proteins to delay Nav inactivation, distal axonal Navs show little FHF association or FHF requirement for high-frequency transmission, velocity and waveforms of conducting action potentials. In addition, leak conductance density along the distal axon is estimated as <1% that of somatodendritic membrane. The faster inactivation rate of FHF-free Navs together with very low axonal leak conductance serves to minimize ionic fluxes and energetic demand during repetitive spike conduction and at rest. The absence of FHFs from Navs at nodes of Ranvier in the central nervous system suggests a similar mechanism of current flux minimization along myelinated axons.
Figures
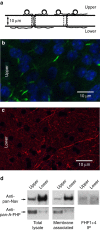
Figure 1. Limited association of FHFs with sodium channels on distal axons of cultured GrCs.
(a) Schematic depiction of culture system. Granule cells dissociated from juvenile mice (P8) are seeded onto the upper surface of hanging culture filter membranes (10 μm thick, 3 μm diameter pores) and cultured for 25 days. Growing axons can traverse pores and continue extension along lower surface. (b) Confocal fluorescence imaging of an upper surface showing TOPRO iodide-stained nuclei (pseudocoloured blue) and short ∼5 μm AISs visualized with anti-AnkyrinG monoclonal antibody (green). (c) Fluorescence imaging of a lower surface showing distal axons detected with neurofilament-A antibody (red) and lacking cell soma (blue). (d) FHF, Nav and FHF/Nav complexes in lysates of total cells and distal axons. Left; 20 μg protein extracted from upper surface (whole cell) and 10 μg protein extracted from lower surface (distal axons) were directly electrophoresed and immunoblotted for detection of sodium channels with pan-Nav antibody and detection of all A-type FHFs with pan-A-FHF antibody. Center; live cultures were surface-biotinylated before extraction and streptavidin-agarose pull-down of labelled proteins, which were then immunoblotted for sodium channels and A-type FHFs. Right; equal amounts of whole cell (upper surface) and distal axon (lower surface) lysates were immunoprecipitated with a combination of antibodies specific for all isoforms of FHF1 and FHF4 followed by immunoblot detection of sodium channels. A much smaller fraction of sodium channels in distal axons show association with any of the FHFs tested. The corresponding uncropped immunoblots are shown in Supplementary Fig. 1.
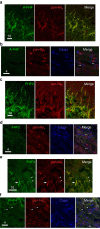
Figure 2. Preferential absence of FHFs from nodes of Ranvier along CNS-myelinated axons.
(a) Immunofluorescence colocalization of A-type FHFs (green) and sodium channels (red) at hippocampal CA1 pyramidal neuron AISs. (b) Absence of A-type FHFs (green) from corpus callosal nodes of Ranvier visualized by sodium channels (red) flanked by paranodal Caspr (blue) (**). Further A-type FHF immunofluorescence is provided in Supplementary Fig. 2. (c) Colocalization of FHF2 (green) and sodium channels (red) at AIS of CA1 pyramidal neurons. (d) Absence of FHF2 (green) from corpus callosal nodes of Ranvier (red) flanked by paranodal Caspr (blue) (**). (e) Colocalization of FHF4 (green) and sodium channels (red) at a cerebellar Purkinje cell AIS (arrow) along with AIS of numerous granule cells (arrowheads). (f) FHF4 (green) is absent from nodes of Ranvier (red) in the cerebellar cortical white matter (**), while present in adjacent granule layer AISs (arrowheads). Scale bars, (a,c,e) 10 μm and (b,d,f) 4 μm.
Figure 3. Fluorescent imaging of action potential conduction along axons of WT and _Fhf1_−/−_Fhf4_−/− cultured GrCs.
(a) High-resolution image composite for WT neuron filled with voltage-sensitive fluorophore JPW3028. Dendrites (arrowheads) and axon (yellow arrows) are indicated. (b) Same cell as in A photographed at 0.2 ms exposure with high-speed, low-resolution camera used for dynamic fluorescence imaging. Coloured pixels are regions along axon monitored during spike conduction. (c) Spike conduction along axon of cell in a,b in response to 0.2 ms 2 nA somatic current pulse (arrow). Fluorescence was sampled at 5 kHz in regions indicated by colours corresponding to b. Stimulation induced spike with Δ_F_ of 6.5% that conducted down axon at ∼0.2 mm ms−1. Data shown are the average from 40 stimulus trials. I=fluorescence intensity, RLI=resting light intensity at the sampled region. (d) High-resolution image composite for _Fhf1_−/−_Fhf4_−/− neuron, with dendrites (arrowheads) and axon (yellow arrows) indicated. (e) Same mutant cell imaged at high-speed and low-resolution. (f) Spike conduction along mutant cell axon (d,e) in response to somatic current pulse (arrow) was indistinguishable in amplitude (beyond 150 μm) and conduction velocity compared with spike from WT cell (c). (g) 60 Hz spike conduction along WT axon. The JPW3028-filled neuron was injected in the soma with biphasic current pulses (700 pA for 1 ms followed by −700 pA for 1 ms) at 60 Hz to induce spike train. Fluorescence was sampled at 2 kHz at different distances along the axon, and data averaged over 15 trials. The spike train faithfully conducted over 230 μm. (h) A total of 60 Hz spike conduction along _Fhf1_−/−_Fhf4_−/− axon. The mutant cell was subjected to same analysis as in g, and showed faithful transmission of the spike train over 250 μm. (i,j) Antidromic spike conduction along axon of _Fhf1_−/−_Fhf4_−/− cell. A mutant granule cell axon was stimulated at 60 Hz at a point 250 μm from the soma, and fluorescence was analyzed at proximal regions, as indicated in high-speed still image (i). All spikes back-propagated towards soma and were detectable in dendrites (j).
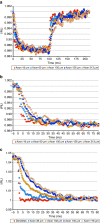
Figure 4. Passive charging of cultured GrC axons in response to somatic voltage clamp.
(a) Axonal response to somatic hyperpolarization. A WT neuron filled with JPW3028 was patched on the soma, held at −60 mV and subjected to a 100 ms −100 mV step followed by step return to −60 mV. Fluorescence was sampled at 2 kHz, and data were averaged over 50 trials. At each axonal position, fluorescence intensity (I) was normalized to that position's resting light intensity (RLI). The voltage step induced very similar fluorescence changes at points ranging from 18 to 213 μm, suggesting comparable charging and limited leak conductance. (b) Expanded time scale of data in a, showing slower charging of axon as function of distance from soma. (c) Passive axonal response to large somatic voltage change. A WT neuron filled with JPW3028 was patched on the soma in the presence of tetrodotoxin, tetraethylamine, 4-amino purine and cadmium to inhibit voltage-gated sodium, potassium and calcium channels. The soma was stepped from −80 to +80 mV for 100 ms followed by return to −80 mV and images collected at 2 kHz intervals. Fluorescence at different points along axon are shown for the repolarization step initiating at _t_=0 ms. All points along axon ranging from 36 to 178 μm undergo very similar change in fluorescence, with longer charging times as function of distance from soma. Data shown are the average of 50 trials.
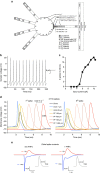
Figure 5. Revised GrC computational model predicts energy benefit of FHF-independent spike conduction.
(a) Schematic of revised model. Clustered sodium channels at hillock and AIS are modelled with associated FHFs ( ), while low-density sodium channels on distal axon and parallel fibres are modelled without FHFs (
), while low-density sodium channels on distal axon and parallel fibres are modelled without FHFs ( ) (see Supplementary Fig. 5 for Nav simulations +/− FHF). Fibre leak conductance (LKG3) is set to 2 mS cm−2 at the AIS (
) (see Supplementary Fig. 5 for Nav simulations +/− FHF). Fibre leak conductance (LKG3) is set to 2 mS cm−2 at the AIS ( ) and to 5 μS cm−2 along distal axon and parallel fibres (
) and to 5 μS cm−2 along distal axon and parallel fibres ( ). Somatic leak conductance (LKG1) combines with inward rectifying potassium conductance (KIR) to set somatic resting membrane potential to −70 mV. The AIS also has elevated axial resistance (500 Ohm*cm,
). Somatic leak conductance (LKG1) combines with inward rectifying potassium conductance (KIR) to set somatic resting membrane potential to −70 mV. The AIS also has elevated axial resistance (500 Ohm*cm,  ) compared with all other cellular compartments (100 Ohm*cm,
) compared with all other cellular compartments (100 Ohm*cm,  ). (b) Somatic spikes simulated in response to 10 pA somatic current injection. Spike frequency is similar to that described in the earlier version of the GrC model. (c) Current-to-spike relationship. Plot of spike number over 240 ms during 2–10 pA simulated current injections. (d) High-frequency spike initiation and propagation. Voltage in soma and axonal and PF compartments (distance from soma indicated) are plotted in response to simulated 8 pA somatic injection generating a 47 Hz spike train. Spikes initiating at AIS conduct down axon and parallel fibres and back-propagate to soma. Parallel fibre spike conduction velocity (0.22 mm ms−1) and 50–50% width (1.0 ms) are within 10% of fluorescence measurements (Fig. 3c,e). (e) Parallel fibre spike currents modelled with or without associated FHFs. Left; sodium (blue) and potassium (red) currents in a parallel fibre compartment during a conducting spike (inset) as in d. On the basis of current fluxes, axoplasm volume, and equation 1 C=10 μmol cations, calculated Na+ influx and K+ efflux from parallel fibre are 0.59 mM per spike. Right; parallel fibres were remodelled with all sodium channels associated with FHF, and Nav and Kv densities were adjusted to preserve shape and amplitude of the conducting spike (inset). Slower Nav inactivation due to FHF increased temporal overlap between Nav and Kv currents, thereby increasing calculated Na+ influx and K+ efflux (0.86 mM) per spike.
). (b) Somatic spikes simulated in response to 10 pA somatic current injection. Spike frequency is similar to that described in the earlier version of the GrC model. (c) Current-to-spike relationship. Plot of spike number over 240 ms during 2–10 pA simulated current injections. (d) High-frequency spike initiation and propagation. Voltage in soma and axonal and PF compartments (distance from soma indicated) are plotted in response to simulated 8 pA somatic injection generating a 47 Hz spike train. Spikes initiating at AIS conduct down axon and parallel fibres and back-propagate to soma. Parallel fibre spike conduction velocity (0.22 mm ms−1) and 50–50% width (1.0 ms) are within 10% of fluorescence measurements (Fig. 3c,e). (e) Parallel fibre spike currents modelled with or without associated FHFs. Left; sodium (blue) and potassium (red) currents in a parallel fibre compartment during a conducting spike (inset) as in d. On the basis of current fluxes, axoplasm volume, and equation 1 C=10 μmol cations, calculated Na+ influx and K+ efflux from parallel fibre are 0.59 mM per spike. Right; parallel fibres were remodelled with all sodium channels associated with FHF, and Nav and Kv densities were adjusted to preserve shape and amplitude of the conducting spike (inset). Slower Nav inactivation due to FHF increased temporal overlap between Nav and Kv currents, thereby increasing calculated Na+ influx and K+ efflux (0.86 mM) per spike.
Similar articles
- SCN5A variant that blocks fibroblast growth factor homologous factor regulation causes human arrhythmia.
Musa H, Kline CF, Sturm AC, Murphy N, Adelman S, Wang C, Yan H, Johnson BL, Csepe TA, Kilic A, Higgins RS, Janssen PM, Fedorov VV, Weiss R, Salazar C, Hund TJ, Pitt GS, Mohler PJ. Musa H, et al. Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):12528-33. doi: 10.1073/pnas.1516430112. Epub 2015 Sep 21. Proc Natl Acad Sci U S A. 2015. PMID: 26392562 Free PMC article. - Autonomous initiation and propagation of action potentials in neurons of the subthalamic nucleus.
Atherton JF, Wokosin DL, Ramanathan S, Bevan MD. Atherton JF, et al. J Physiol. 2008 Dec 1;586(23):5679-700. doi: 10.1113/jphysiol.2008.155861. Epub 2008 Oct 2. J Physiol. 2008. PMID: 18832425 Free PMC article. - Sodium Channel Nav1.8 Underlies TTX-Resistant Axonal Action Potential Conduction in Somatosensory C-Fibers of Distal Cutaneous Nerves.
Klein AH, Vyshnevska A, Hartke TV, De Col R, Mankowski JL, Turnquist B, Bosmans F, Reeh PW, Schmelz M, Carr RW, Ringkamp M. Klein AH, et al. J Neurosci. 2017 May 17;37(20):5204-5214. doi: 10.1523/JNEUROSCI.3799-16.2017. Epub 2017 Apr 27. J Neurosci. 2017. PMID: 28450535 Free PMC article. - Molecular dissection of the myelinated axon.
Waxman SG, Ritchie JM. Waxman SG, et al. Ann Neurol. 1993 Feb;33(2):121-36. doi: 10.1002/ana.410330202. Ann Neurol. 1993. PMID: 7679565 Review. - Signal propagation along the axon.
Rama S, Zbili M, Debanne D. Rama S, et al. Curr Opin Neurobiol. 2018 Aug;51:37-44. doi: 10.1016/j.conb.2018.02.017. Epub 2018 Mar 8. Curr Opin Neurobiol. 2018. PMID: 29525575 Review.
Cited by
- Parameter tuning differentiates granule cell subtypes enriching transmission properties at the cerebellum input stage.
Masoli S, Tognolina M, Laforenza U, Moccia F, D'Angelo E. Masoli S, et al. Commun Biol. 2020 May 8;3(1):222. doi: 10.1038/s42003-020-0953-x. Commun Biol. 2020. PMID: 32385389 Free PMC article. - Single Neuron Optimization as a Basis for Accurate Biophysical Modeling: The Case of Cerebellar Granule Cells.
Masoli S, Rizza MF, Sgritta M, Van Geit W, Schürmann F, D'Angelo E. Masoli S, et al. Front Cell Neurosci. 2017 Mar 15;11:71. doi: 10.3389/fncel.2017.00071. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28360841 Free PMC article. - Hebbian Spike-Timing Dependent Plasticity at the Cerebellar Input Stage.
Sgritta M, Locatelli F, Soda T, Prestori F, D'Angelo EU. Sgritta M, et al. J Neurosci. 2017 Mar 15;37(11):2809-2823. doi: 10.1523/JNEUROSCI.2079-16.2016. Epub 2017 Feb 10. J Neurosci. 2017. PMID: 28188217 Free PMC article. - The Axon Initial Segment: An Updated Viewpoint.
Leterrier C. Leterrier C. J Neurosci. 2018 Feb 28;38(9):2135-2145. doi: 10.1523/JNEUROSCI.1922-17.2018. Epub 2018 Jan 29. J Neurosci. 2018. PMID: 29378864 Free PMC article. Review. - Challenges and Perspectives of Quantitative Functional Sodium Imaging (fNaI).
Gandini Wheeler-Kingshott CAM, Riemer F, Palesi F, Ricciardi A, Castellazzi G, Golay X, Prados F, Solanky B, D'Angelo EU. Gandini Wheeler-Kingshott CAM, et al. Front Neurosci. 2018 Nov 9;12:810. doi: 10.3389/fnins.2018.00810. eCollection 2018. Front Neurosci. 2018. PMID: 30473659 Free PMC article.
References
- Häusser M., Stuart G., Racca C. & Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron 15, 637–647 (1995). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases