Host Cellular Protein TRAPPC6AΔ Interacts with Influenza A Virus M2 Protein and Regulates Viral Propagation by Modulating M2 Trafficking - PubMed (original) (raw)
. 2016 Dec 16;91(1):e01757-16.
doi: 10.1128/JVI.01757-16. Print 2017 Jan 1.
Libin Liang 1, Xinyuan Shao 1, Weiyu Luo 1, Shuitao Jiang 1, Qingqing Zhao 1, Nan Sun 1, Yuhui Zhao 1, Junping Li 1, Jinguang Wang 1, Yuan Zhou 1, Jie Zhang 1, Guangwen Wang 1, Li Jiang 1, Hualan Chen 2, Chengjun Li 2
Affiliations
- PMID: 27795429
- PMCID: PMC5165196
- DOI: 10.1128/JVI.01757-16
Host Cellular Protein TRAPPC6AΔ Interacts with Influenza A Virus M2 Protein and Regulates Viral Propagation by Modulating M2 Trafficking
Pengyang Zhu et al. J Virol. 2016.
Abstract
Influenza A virus (IAV) matrix protein 2 (M2) plays multiple roles in the early and late phases of viral infection. Once synthesized, M2 is translocated to the endoplasmic reticulum (ER), travels to the Golgi apparatus, and is sorted at the trans-Golgi network (TGN) for transport to the apical plasma membrane, where it functions in virus budding. We hypothesized that M2 trafficking along with its secretory pathway must be finely regulated, and host factors could be involved in this process. However, no studies examining the role of host factors in M2 posttranslational transport have been reported. Here, we used a yeast two-hybrid (Y2H) system to screen for host proteins that interact with the M2 protein and identified transport protein particle complex 6A (TRAPPC6A) as a potential binding partner. We found that both TRAPPC6A and its N-terminal internal-deletion isoform, TRAPPC6A delta (TRAPPC6AΔ), interact with M2. Truncation and mutation analyses showed that the highly conserved leucine residue at position 96 of M2 is critical for mediating this interaction. The role of TRAPPC6AΔ in the viral life cycle was investigated by the knockdown of endogenous TRAPPC6AΔ with small interfering RNA (siRNA) and by generating a recombinant virus that was unable to interact with TRAPPC6A/TRAPPC6AΔ. The results indicated that TRAPPC6AΔ, through its interaction with M2, slows M2 trafficking to the apical plasma membrane, favors viral replication in vitro, and positively modulates virus virulence in mice.
Importance: The influenza A virus M2 protein regulates the trafficking of not only other proteins but also itself along the secretory pathway. However, the host factors involved in the regulation of the posttranslational transport of M2 are largely unknown. In this study, we identified TRAPPC6A and its N-terminal internal-deletion isoform, TRAPPC6AΔ, as interacting partners of M2. We found that the leucine (L) residue at position 96 of M2 is critical for mediating this interaction, which leads us to propose that the high level of conservation of 96L is a consequence of M2 adaptation to its interacting host factor TRAPPC6A/TRAPPC6AΔ. Importantly, we discovered that TRAPPC6AΔ can positively regulate viral replication in vitro by modulating M2 trafficking to the plasma membrane.
Keywords: M2; TRAPPC6AΔ; influenza A virus; transport.
Copyright © 2016 Zhu et al.
Figures
FIG 1
Protein-protein interaction of IAV M2 with TRAPPC6A in the Y2H system. Yeast strain Y2HGold was cotransformed with one of the bait plasmids (BD-AH05M2, BD-SC09M2, BD-AH05M2CT, or BD-SC09M2CT), together with the prey plasmid AD-TRAPPC6A, which encodes TRAPPC6A fused to the Gal4 activation domain. Positive protein-protein interactions are indicated by the appearance of blue colonies in the presence of X-α-Gal. Cotransformation of pGBKT7-53 encoding the Gal4 DNA-BD fused with murine p53 (BD-p53) and pGADT7-T encoding the Gal4 activation domain fused with the simian virus 40 large T antigen (AD-T) served as a positive control, since p53 and large T antigen are known to interact in the Y2H system. Cotransformation of pGBKT7-Lam, which encodes the Gal4-BD fused with human lamin C (BD-Lam), and AD-T served as a negative control, since lamin C does not form complexes or interact with most other proteins. DDO, SD/−Leu/−Trp; QDO, SD/−Ade/−His/−Leu/−Trp; QDO/X/A, SD/−Ade/−His/−Leu/−Trp/X-α-Gal/AbA.
FIG 2
M2 interacts with TRAPPC6A and TRAPPC6AΔ in mammalian cells. (A to C) Plasmids expressing TRAPPC6A-Myc and Flag-SC09M2 (A), TRAPPC6A-Myc and Flag-AH05M2 (B), or TRAPPC6A-Myc and Flag-WSNM2 (C) were transfected individually or in combination, as indicated, in HEK293T cells. Forty-eight hours after transfection, cell lysates were immunoprecipitated with a mouse anti-Flag MAb or a mouse anti-Myc MAb and were subjected to Western blotting with a rabbit anti-Flag polyclonal antibody or a rabbit anti-Myc polyclonal antibody to reveal the presence of M2 and TRAPPC6A, respectively. (D) Western blotting of proteins bound to GST alone or to GST-TRAPPC6A. HEK293T cells transfected with pCAGGS-SC09M2 or with the pCAGGS vector were lysed with IP buffer, and the lysate was incubated with purified GST or GST-TRAPPC6A and then subjected to a pulldown assay. Equal volumes of proteins bound to the beads and the original cell lysates (5% of the input) were examined by Western blotting using a mouse anti-M2 MAb or a mouse anti-actin MAb, respectively. The GST-tagged proteins in the eluates were detected by Coomassie blue (CB) staining. (E) Confocal analysis of the distribution of M2 and TRAPPC6A proteins in A549 cells. pCAGGS-TRAPPC6A-myc and pCAGGS-Flag-SC09M2 were transfected individually or in combination into A549 cells and assessed by immunofluorescence staining. IAV M2 was detected with a mouse anti-Flag MAb and visualized with Alexa Fluor 488 (green). TRAPPC6A was detected with a rabbit anti-Myc polyclonal antibody and visualized with Alexa Fluor 546 (red). Yellow indicates colocalization of Alexa Fluor 546 and 488 in the merged image. (F) pCAGGS-TRAPPC6A-myc was cotransfected with pEGFP-C1 or pEGFP-C1-BM2 into HEK293T cells for 48 h before the cells were lysed. Following immunoprecipitation of the cell lysates with a mouse anti-GFP MAb, the immunoprecipitates were analyzed by Western blotting using a rabbit anti-GFP polyclonal antibody or a rabbit anti-Myc polyclonal antibody to reveal the presence of BM2 and TRAPPC6A, respectively. (G) Co-IP of M2 and TRAPPC6AΔ. pCAGGS-Flag-SC09M2 was cotransfected with pCAGGS-TRAPPC6A-myc or pCAGGS-TRAPPC6AΔ-myc into HEK293T cells. Forty-eight hours after transfection, cell lysates were immunoprecipitated with a mouse anti-Myc MAb and subjected to Western blotting with a rabbit anti-Flag polyclonal antibody or a rabbit anti-Myc polyclonal antibody to reveal the presence of M2 and TRAPPC6A or TRAPPC6AΔ, respectively.
FIG 3
A leucine residue at position 96 of M2 is required for the TRAPPC6A interaction. (A) pCAGGS-TRAPPC6A-myc was cotransfected with pEGFP-C1, pEGFP-C1-SC09 M2, pEGFP-C1-SC09 M2EDTM, or pEGFP-C1-SC09 M2CT into HEK293T cells for 48 h before preparation for cell lysates. Following immunoprecipitation with a mouse anti-GFP MAb, the immunoprecipitates were analyzed by Western blotting using a rabbit anti-GFP polyclonal antibody and a rabbit anti-Myc polyclonal antibody to reveal the presence of M2 and TRAPPC6A, respectively. (B and C) Plasmids expressing TRAPPC6A-myc and Flag-SC09M2 or Flag-SC09M2 with different amino acid deletions in the C terminus were cotransfected into HEK293T cells for 48 h before the preparation of cell lysates. Following immunoprecipitation with a mouse anti-Flag MAb, the immunoprecipitates were analyzed by Western blotting using a rabbit anti-Flag polyclonal antibody or a rabbit anti-Myc polyclonal antibody to reveal the presence of M2 and TRAPPC6A, respectively.
FIG 4
Mutation at position 96 of M2 affects its interaction with TRAPPC6A. (A) Sequence analysis of IAV M2 at position 96. All of the IAV M2 sequences deposited in GenBank by 6 July 2014 were downloaded. The identity of the amino acids at position 96 was statistically analyzed. (B) Plasmids expressing TRAPPC6A-Myc and Flag-WSNM2 or Flag-WSNM2 with different mutations at position 96 were cotransfected into HEK293T cells for 48 h before the preparation of cell lysates. Following immunoprecipitation with a mouse anti-Flag MAb, the immunoprecipitates were analyzed by Western blotting using a rabbit anti-Flag polyclonal antibody or a rabbit anti-Myc polyclonal antibody to reveal the presence of M2 and TRAPPC6A, respectively.
FIG 5
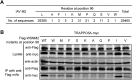
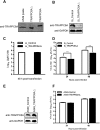
TRAPPC6AΔ positively modulates influenza virus infection. (A) Endogenous expression of TRAPPC6AΔ in A549 cells. Whole lysates of A549 cells grown in 12-well plates were subjected to Western blotting with a rabbit anti-TRAPPC6A polyclonal antibody. HEK293T cell lysates transiently transfected with pCAGGS-TRAPPC6A or pCAGGS-TRAPPC6AΔ were used as a control. (B) siRNA knockdown of TRAPPC6AΔ in A549 cells. A549 cells were transfected with siRNA targeting TRAPPC6AΔ or nontargeting siRNA for 48 h. Whole-cell lysates were then collected and analyzed by Western blotting with a rabbit anti-TRAPPC6A polyclonal antibody. (C) Cell viability of siRNA-treated A549 cells measured by using the CellTiter-Glo assay. A549 cells were transfected with siRNA as described above for panel B. The data are presented as means ± standard deviations for triplicate transfections. (D) Virus replication in siRNA-treated A549 cells. Cells transfected with siRNA as described above for panel B were infected with WSN virus. At 24 and 48 h p.i., supernatants were collected and titrated for infectious virus by plaque assays in MDCK cells. Three independent experiments were performed, and data are shown as means ± standard deviations for triplicates from a representative experiment. **, P < 0.01. (E) Stable A549 cell line overexpressing TRAPPC6AΔ. A549 cells were used to establish a stable cell line overexpressing TRAPPC6AΔ by using a retroviral vector. The stable overexpression of TRAPPC6AΔ was confirmed by Western blotting with a rabbit anti-TRAPPC6A polyclonal antibody in comparison with the A549 control cell line transduced with an empty retrovirus. (F) Virus replication in TRAPPC6AΔ-overexpressing A549 cells. WSN virus was used to infect the TRAPPC6AΔ-overexpressing A549 cell line or the A549 control cell line transduced with the empty retrovirus at an MOI of 0.01. Supernatants were collected at 24 and 48 h p.i., and virus titers were determined by plaque assays on MDCK cells. Three independent experiments were performed, and data are shown as means ± standard deviations for triplicates from a representative experiment.
FIG 6
Loss of the interaction between M2 and TRAPPC6A/TRAPPC6AΔ attenuates influenza virus in vitro and in vivo. (A) Generation of WSN viruses with M2 deletions that prevent the interaction with TRAPPC6A/TRAPPC6AΔ by using reverse genetics. HEK293T cells were transfected with plasmids for the synthesis of eight vRNAs, among which the M vRNA segment encoded wt M2 or M2 mutants lacking 1 or 2 amino acids from the C terminus, together with the four supporting protein expression plasmids. The transfection supernatants were harvested at 48 h posttransfection and titrated by plaque assays in MDCK cells. Three independent experiments were performed, and data are shown as means ± standard deviations for triplicates from a representative experiment. **, P < 0.01. (B) Growth kinetics of M2 deletion mutant viruses in A549 cells. A549 cells were infected with the indicated viruses at an MOI of 0.01, and the supernatants were collected at various times and titrated by plaque assays in MDCK cells. Three independent experiments were performed, and the data shown are from a representative experiment. (C) Growth kinetics of M2 deletion mutant viruses in TRAPPC6AΔ-knocked-down A549 cells. A549 cells treated with siRNA targeting TRAPPC6AΔ for 48 h were infected with the indicated viruses at an MOI of 0.01, and the supernatants were collected at various times and titrated by plaque assays in MDCK cells. Three independent experiments were performed; the data shown are from a representative experiment. (D) Pathogenesis of M2 deletion mutant viruses in vivo. Mice were inoculated intranasally with the indicated viruses, and mortality was observed for 14 days after inoculation.
FIG 7
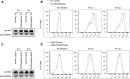
Effect of modulation of TRAPPC6AΔ expression on the cell surface expression of viral and cellular proteins. (A) A549 cells were transfected with siRNA targeting TRAPPC6AΔ or with nontargeting siRNA for 48 h and were then infected with the WSN virus at an MOI of 3. Cell lysates were processed at 8 and 10 h p.i. and subjected to Western blotting using a mouse anti-M2 MAb to detect the expression level of M2. (B) A549 cells were treated with siRNA and infected with the WSN virus as described above for panel A. Cells were fixed at 8 and 10 h p.i., left nonpermeabilized, and stained with the mouse anti-M2 MAb and Alexa Fluor 488-conjugated donkey anti-mouse IgG(H+L) for M2 surface expression analysis by flow cytometry. The graph shows the fluorescence intensity of M2 surface expression. (C) The TRAPPC6AΔ-overexpressing A549 cell line or the A549 control cell line transduced with an empty retrovirus was infected with the WSN virus at an MOI of 3. Cell lysates were processed at 8 and 10 h p.i. and subjected to Western blotting using a mouse anti-M2 MAb to detect the expression level of M2. (D) The TRAPPC6AΔ-overexpressing A549 cell line or the A549 control cell line transduced with an empty retrovirus was infected with the WSN virus at an MOI of 3. The cell surface expression of M2 was analyzed by flow cytometry at 8 and 10 h p.i. as described above for panel B. (E) A549 cells were treated with siRNA and infected with the WSN virus as described above for panel A. The cell surface expression of HA was analyzed by flow cytometry at 8 and 10 h p.i. as described above for panel B by using the rabbit anti-HA polyclonal antibody and Alexa Fluor 488-conjugated goat anti-rabbit IgG(H+L). (F) A549 cells were treated with siRNA and infected with the WSN virus as described above for panel A. The cell surface expression of FGF2 was analyzed by flow cytometry at 8 and 10 h p.i. as described above for panel B by using the rabbit anti-FGF2 MAb and Alexa Fluor 488-conjugated goat anti-rabbit IgG(H+L). (G) A549 cells were treated with siRNA and infected with the WSN virus as described above for panel A. At 2 h p.i., the culture medium was replaced with medium supplemented with 25 μM amantadine. The cell surface expression of M2 was analyzed by flow cytometry at 8 and 10 h p.i. as described above for panel B.
FIG 7
Effect of modulation of TRAPPC6AΔ expression on the cell surface expression of viral and cellular proteins. (A) A549 cells were transfected with siRNA targeting TRAPPC6AΔ or with nontargeting siRNA for 48 h and were then infected with the WSN virus at an MOI of 3. Cell lysates were processed at 8 and 10 h p.i. and subjected to Western blotting using a mouse anti-M2 MAb to detect the expression level of M2. (B) A549 cells were treated with siRNA and infected with the WSN virus as described above for panel A. Cells were fixed at 8 and 10 h p.i., left nonpermeabilized, and stained with the mouse anti-M2 MAb and Alexa Fluor 488-conjugated donkey anti-mouse IgG(H+L) for M2 surface expression analysis by flow cytometry. The graph shows the fluorescence intensity of M2 surface expression. (C) The TRAPPC6AΔ-overexpressing A549 cell line or the A549 control cell line transduced with an empty retrovirus was infected with the WSN virus at an MOI of 3. Cell lysates were processed at 8 and 10 h p.i. and subjected to Western blotting using a mouse anti-M2 MAb to detect the expression level of M2. (D) The TRAPPC6AΔ-overexpressing A549 cell line or the A549 control cell line transduced with an empty retrovirus was infected with the WSN virus at an MOI of 3. The cell surface expression of M2 was analyzed by flow cytometry at 8 and 10 h p.i. as described above for panel B. (E) A549 cells were treated with siRNA and infected with the WSN virus as described above for panel A. The cell surface expression of HA was analyzed by flow cytometry at 8 and 10 h p.i. as described above for panel B by using the rabbit anti-HA polyclonal antibody and Alexa Fluor 488-conjugated goat anti-rabbit IgG(H+L). (F) A549 cells were treated with siRNA and infected with the WSN virus as described above for panel A. The cell surface expression of FGF2 was analyzed by flow cytometry at 8 and 10 h p.i. as described above for panel B by using the rabbit anti-FGF2 MAb and Alexa Fluor 488-conjugated goat anti-rabbit IgG(H+L). (G) A549 cells were treated with siRNA and infected with the WSN virus as described above for panel A. At 2 h p.i., the culture medium was replaced with medium supplemented with 25 μM amantadine. The cell surface expression of M2 was analyzed by flow cytometry at 8 and 10 h p.i. as described above for panel B.
FIG 8
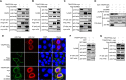
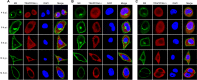
Dynamics of the interaction of M2 and TRAPPC6AΔ in wt and mutant WSN virus-infected cells. A549 cells were infected with wt influenza virus WSN (A), or one of the M2 deletion mutants WSN M2Del1 (B) and WSN M2Del2 (C), at an MOI of 5. At 4, 6, 8, 10, and 14 h p.i., the infected cells were fixed and stained with mouse anti-M2 MAb 14C2 and rabbit anti-TRAPPC6A polyclonal antibody, followed by incubation with Alexa Fluor 488 donkey anti-mouse IgG(H+L) (green) and Alexa Fluor 546 donkey anti-rabbit IgG(H+L) (red). Nuclei were stained with DAPI.
FIG 9
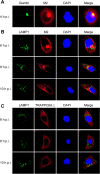
Confocal microscopy of WSN virus-infected cells stained for the Golgi apparatus or lysosomes. A549 cells were infected with the wt WSN virus at an MOI of 5. At the indicated time points, infected cells were fixed and stained with mouse anti-Giantin MAb and rabbit anti-M2 polyclonal antibody (A), mouse anti-LAMP1 MAb and rabbit anti-M2 polyclonal antibody (B), or mouse anti-LAMP1 MAb and rabbit anti-TRAPPC6A polyclonal antibody (C), followed by incubation with Alexa Fluor 488 donkey anti-mouse IgG(H+L) (green) and Alexa Fluor 546 donkey anti-rabbit IgG(H+L) (red). Nuclei were stained with DAPI.
Similar articles
- Influenza A Virus M2 Protein Apical Targeting Is Required for Efficient Virus Replication.
Wohlgemuth N, Lane AP, Pekosz A. Wohlgemuth N, et al. J Virol. 2018 Oct 29;92(22):e01425-18. doi: 10.1128/JVI.01425-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30158290 Free PMC article. - Influenza virus matrix protein M1 interacts with SLD5 to block host cell cycle.
Zhu L, Zhao W, Lu J, Li S, Zhou K, Jiang W, Duan X, Fu L, Yu B, Cai KQ, Gao GF, Liu W, Fang M. Zhu L, et al. Cell Microbiol. 2019 Aug;21(8):e13038. doi: 10.1111/cmi.13038. Epub 2019 May 16. Cell Microbiol. 2019. PMID: 31050118 - The role of nuclear NS1 protein in highly pathogenic H5N1 influenza viruses.
Mok BW, Liu H, Chen P, Liu S, Lau SY, Huang X, Liu YC, Wang P, Yuen KY, Chen H. Mok BW, et al. Microbes Infect. 2017 Dec;19(12):587-596. doi: 10.1016/j.micinf.2017.08.011. Epub 2017 Sep 10. Microbes Infect. 2017. PMID: 28903072 - Host microRNAs and exosomes that modulate influenza virus infection.
Zheng B, Zhou J, Wang H. Zheng B, et al. Virus Res. 2020 Apr 2;279:197885. doi: 10.1016/j.virusres.2020.197885. Epub 2020 Jan 22. Virus Res. 2020. PMID: 31981772 Review. - [Functional analysis of Host proteins involved in RNA virus replication].
Yamayoshi S. Yamayoshi S. Uirusu. 2018;68(1):71-78. doi: 10.2222/jsv.68.71. Uirusu. 2018. PMID: 31105137 Review. Japanese.
Cited by
- Cholesterol and M2 Rendezvous in Budding and Scission of Influenza A Virus.
Madsen JJ, Rossman JS. Madsen JJ, et al. Subcell Biochem. 2023;106:441-459. doi: 10.1007/978-3-031-40086-5_16. Subcell Biochem. 2023. PMID: 38159237 - Viroporins in the Influenza Virus.
To J, Torres J. To J, et al. Cells. 2019 Jun 29;8(7):654. doi: 10.3390/cells8070654. Cells. 2019. PMID: 31261944 Free PMC article. Review. - MARCH8 inhibits influenza A virus infection by targeting viral M2 protein for ubiquitination-dependent degradation in lysosomes.
Liu X, Xu F, Ren L, Zhao F, Huang Y, Wei L, Wang Y, Wang C, Fan Z, Mei S, Song J, Zhao Z, Cen S, Liang C, Wang J, Guo F. Liu X, et al. Nat Commun. 2021 Jul 20;12(1):4427. doi: 10.1038/s41467-021-24724-2. Nat Commun. 2021. PMID: 34285233 Free PMC article. - YWHAG inhibits influenza a virus replication by suppressing the release of viral M2 protein.
Mao H, Cao L, Xu T, Xia X, Ren P, Han P, Li C, Hui X, Lin X, Huang K, Jin M. Mao H, et al. Front Microbiol. 2022 Jul 19;13:951009. doi: 10.3389/fmicb.2022.951009. eCollection 2022. Front Microbiol. 2022. PMID: 35928168 Free PMC article. - TRIM35 mediates protection against influenza infection by activating TRAF3 and degrading viral PB2.
Sun N, Jiang L, Ye M, Wang Y, Wang G, Wan X, Zhao Y, Wen X, Liang L, Ma S, Liu L, Bu Z, Chen H, Li C. Sun N, et al. Protein Cell. 2020 Dec;11(12):894-914. doi: 10.1007/s13238-020-00734-6. Epub 2020 Jun 19. Protein Cell. 2020. PMID: 32562145 Free PMC article.
References
- Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. 2012. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 337:199–204. doi:10.1126/science.1222213. - DOI - PMC - PubMed
- Wise HM, Hutchinson EC, Jagger BW, Stuart AD, Kang ZH, Robb N, Schwartzman LM, Kash JC, Fodor E, Firth AE, Gog JR, Taubenberger JK, Digard P. 2012. Identification of a novel splice variant form of the influenza A virus M2 ion channel with an antigenically distinct ectodomain. PLoS Pathog 8:e1002998. doi:10.1371/journal.ppat.1002998. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous