Dysfunctional MnSOD leads to redox dysregulation and activation of prosurvival AKT signaling in uterine leiomyomas - PubMed (original) (raw)
Dysfunctional MnSOD leads to redox dysregulation and activation of prosurvival AKT signaling in uterine leiomyomas
Vania Vidimar et al. Sci Adv. 2016.
Abstract
AKT signaling promotes cell growth and survival and is often dysregulated via multiple mechanisms in different types of cancer, including uterine leiomyomas (ULMs). ULMs are highly prevalent fibrotic tumors that arise from the smooth muscular layer of the uterus, the myometrium (MM). ULMs pose a major public health issue because they can cause severe morbidity and poor pregnancy outcomes. We investigate the mechanisms driving ULM growth and survival via aberrant activation of AKT. We demonstrate that an acetylation-mediated impairment of the manganese superoxide dismutase (MnSOD) activity is prevalent in ULM cells compared to the normal-matched MM from the same patients. This impairment increases the levels of superoxide and oxidative stress, which activate AKT via oxidative inactivation of the phosphatase and tensin homolog deleted on chromosome 10 (PTEN). Redox activation of AKT promotes ULM cell survival under conditions of moderate but persistent oxidative stress that are compatible with ULM's prooxidative microenvironment. Moreover, because of impaired MnSOD activity, ULM cells are sensitive to high levels of reactive oxygen species (ROS) and superoxide-generating compounds, resulting in decreased ULM cell viability. On the contrary, MM cells with functional MnSOD are more resistant to high levels of oxidants. This study demonstrates a causative role of acetylation-mediated MnSOD dysfunction in activating prosurvival AKT signaling in ULMs. The specific AKT and redox states of ULM cells provide a potential novel therapeutic rationale to selectively target ULM cells because of their defective ROS-scavenging system..
Keywords: AKT; MnSOD; ROS; fibroids; leiomyoma.
Figures
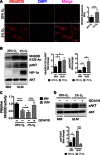
Fig. 1. ULMs exhibit increased levels of acetylated MnSOD and 3-NO as well as decreased MnSOD activity.
(A) Representative images of the TMA containing 60 matched MM and ULM specimens immunostained with MnSOD K122-Ac, MnSOD, and 3-NO antibodies. For each human specimen, two tissue cores were derived from MM and three were derived from ULM. (B) Score frequency distribution of TMA immunostaining. The intensity of well-preserved matched MM/ULM tissue cores was scored numerically as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). Statistically significant differences between normal MM and ULM were evaluated using a χ2 test (***P = 0.0009, ****P < 0.0001; ns, not significant). (C) MnSOD activity was assessed in 13 untreated patient-derived MM and matched ULM cells. Each data point represents the means ± SD of a quadruplicate measurement. Data are represented as fold change to untreated MM cells for each patient (*P < 0.0001; a_P_ = 0.0183; b_P_ = 0.0046; c_P_ = 0.0008; d_P_ = 0.0025; e_P_ = 0.0035; paired t test).
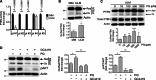
Fig. 2. Mitochondrial ROS derived from dysfunctional acetylated MnSOD lead to AKT activation in ULM cells.
(A) Levels of MnSOD K122-Ac, MnSOD, and pAKT(S473) (pAKT) proteins from cell lysates of untreated MM and ULM cells. pan-AKT (AKT) and actin were used as loading controls. (B and C) ULM and MM cells were exposed to increasing concentrations of PQ or H2O2 for 6 hours, and cellular protein extracts were analyzed by Western blot for pAKT(S473) and AKT. A representative Western blot and the means ± SD of densitometric quantification of three independent experiments are shown [(B) *P < 0.05, ***P < 0.001 versus PQ 0 μM ULM; (C) *P < 0.05, ***P < 0.001 versus H2O2 0 μM ULM; one-way analysis of variance (ANOVA), n = 3]. (D) ULM cells were transiently infected with a lenti-CTR or a lenti–MnSOD K122-R. MnSOD activity (*P = 0.0286; unpaired t test) as well as MnSOD and pAKT protein levels were analyzed, and corresponding densitometric analysis is shown. Data are representative of three patients. (E) ULM cells were treated with 10 μM GC4419 for 6 hours, and pAKT levels were analyzed. AKT was used as loading control. Corresponding densitometric analysis of three independent experiments is shown as means ± SD (**P = 0.0045; unpaired t test, n = 3). (F) ULM and MM cells were treated for 24 hours with increasing doses of PQ or H2O2, and cell viability was measured using WST-1 assay (*P < 0.05, ****P < 0.0001; one-way ANOVA). Results are expressed as means ± SD from three independent experiments (n = 3).
Fig. 3. ULM cells are characterized by aberrant MnSOD acetylation and AKT activation under normoxic and hypoxic conditions.
(A) MitoSOX Red was used to assess mitochondrial superoxide levels in ULM cells under normoxic (20% O2) and hypoxic (2% O2) conditions after 48 hours of incubation time. MitoSOX fluorescence was quantified by analyzing the fluorescence intensity of more than 10 cells for each condition using ImageJ software (****P < 0.0001; one-way ANOVA, n = 3 independent experiments ± SD). RFU, relative fluorescence unit. (B) ULM cells were grown under normoxia and hypoxia for 48 hours, and levels of MnSOD K122-Ac and pAKT(S473) were analyzed. MM cells grown in normoxia were used as control. Hypoxia was verified by detection of HIF-1α. Equal loading was confirmed by anti-AKT. Corresponding densitometric analysis of three independent experiments is shown as means ± SD (*P < 0.05; one-way ANOVA, n = 3). (C and D) MM and ULM cells were grown under both normoxic and hypoxic atmosphere for a total of 48 hours. Twenty-four hours before harvesting, the MnSOD mimetic GC4419 (10 μM) was added to ULM cells. (C) MnSOD activity was assessed as previously described. Data are expressed as means ± SD from three independent experiments (n = 3). (D) pAKT and AKT levels were analyzed by Western blotting, and densitometric analysis of three independent experiments is shown as means ± SD (*P < 0.05, ***P < 0.001, ****P < 0.0001; one-way ANOVA, n = 3).
Fig. 4. PTEN activity is reduced in ULM cells as a result of superoxide-dependent oxidation.
(A) PTEN phosphatase activity was measured in immunoprecipitated PTEN samples from five patient-derived MM and ULM cells using PIP3 as the substrate. Data are represented as fold change to untreated MM cells (paired t test). (B) Protein extracts from untreated MM and ULM cells were analyzed under nonreducing conditions and immunoblotted with anti-PTEN antibody (*P = 0.0162; unpaired t test). (C) ULM cells were treated for 6 hours with 10, 100, 500, and 1000 μM PQ in serum-free medium, and protein extracts were analyzed as described above for oxidized and total PTEN (**P < 0.01, ****P < 0.0001; one-way ANOVA). (D) ULM cells were treated with 100 μM PQ, 10 μM GC4419, or a combination of both drugs for 6 hours. Protein extracts were analyzed under nonreducing conditions and immunoblotted with anti-PTEN antibody (*P < 0.05, **P < 0.01; one-way ANOVA) or under reducing conditions and immunoblotted with pAKT antibody (*P < 0.05, **P < 0.01; one-way ANOVA). For all Western blots in (B) to (D), one representative blot and corresponding densitometric analysis of three independent experiments are shown as means ± SD (n = 3). Anti-actin antibody was used as loading control.
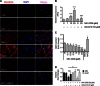
Fig. 5. Treatment with the AKT inhibitor MK-2206 leads to superoxide generation in ULM cells.
(A) Mitochondrial superoxide levels were assessed in ULM cells using MitoSOX Red. ULM cells were treated with vehicle (CTR), various concentrations of MK-2206 (MK; 1, 10, and 25 μM) and 10 μM GC4419 (GC) alone or with 25 μM MK-2206 (GC+MK25) for 6 hours in serum-free media. Representative pictures from three independent experiments are shown. (B) MitoSOX fluorescence was quantified by analyzing the fluorescence intensity of more than 10 cells for each condition using ImageJ software (****P < 0.0001 versus 0 μM MK-2205; ####P < 0.0001 versus 25 μM MK-2205; one-way ANOVA, n = 3 independent experiments ± SD). (C) GSH content was quantified using GSH-Glo reagent. ULM cells were treated at the indicated concentrations of MK-2206 for 24 hours. BSO (10 μM) and 50 μM NAC were used as negative and positive controls, respectively [*P < 0.05, ***P < 0.001, ****P < 0.0001 versus vehicle (dimethyl sulfoxide); one-way ANOVA, n = 3 independent experiments ± SD]. (D) MM and ULM cells were treated with 25 μM MK-2206 alone or in combination with 10 μM GC4419 for 24 hours. Cell viability was determined using WST-1 (**P < 0.01, ****P < 0.0001; one-way ANOVA, n = 3 independent experiments ± SD).
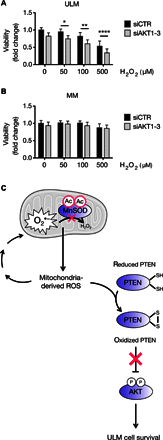
Fig. 6. AKT protects ULM cells from oxidative-induced damage.
(A and B) AKT1, AKT2, and AKT3 were silenced in ULM and MM cells by reverse transfection using siAKT1, siAKT2, and siAKT3. Following AKT knockdown, 50, 100, or 500 μM H2O2 was added to ULM and MM cells for 6 hours, and cell viability was determined using WST-1. Data are shown as means ± SD from three independent experiments (*P < 0.05, **P < 0.01, ****P < 0.0001; one-way ANOVA, n = 3). (C) Proposed working model for the interplay between dysfunctional MnSOD and activation of the AKT pathway and its effects on ULM cell survival. In ULM cells, acetylation of MnSOD impairs its activity, leading to the increase of mitochondrial ROS, which, in turn, activate AKT through oxidative inactivation of PTEN and promote cell survival in the prooxidative ULM microenvironment. The mitochondrion in the figure was taken from the Servier Medical Art database (
http://servier.com/Powerpoint-image-bank
).
Similar articles
- Oxidative stress-induced miRNAs modulate AKT signaling and promote cellular senescence in uterine leiomyoma.
Xu X, Kim JJ, Li Y, Xie J, Shao C, Wei JJ. Xu X, et al. J Mol Med (Berl). 2018 Oct;96(10):1095-1106. doi: 10.1007/s00109-018-1682-1. Epub 2018 Aug 10. J Mol Med (Berl). 2018. PMID: 30097674 Free PMC article. - PI3K/Akt/mTOR signaling & its regulator tumour suppressor genes PTEN & LKB1 in human uterine leiomyomas.
Makker A, Goel MM, Mahdi AA, Bhatia V, Das V, Agarwal A, Pandey A. Makker A, et al. Indian J Med Res. 2016 May;143(Supplement):S112-S119. doi: 10.4103/0971-5916.191808. Indian J Med Res. 2016. PMID: 27748285 Free PMC article. - The AKT/BCL-2 Axis Mediates Survival of Uterine Leiomyoma in a Novel 3D Spheroid Model.
Vidimar V, Chakravarti D, Bulun SE, Yin P, Nowak R, Wei JJ, Kim JJ. Vidimar V, et al. Endocrinology. 2018 Mar 1;159(3):1453-1462. doi: 10.1210/en.2017-03191. Endocrinology. 2018. PMID: 29381777 Free PMC article. - Redox regulation of tumor suppressor PTEN in cancer and aging (Review).
Kitagishi Y, Matsuda S. Kitagishi Y, et al. Int J Mol Med. 2013 Mar;31(3):511-5. doi: 10.3892/ijmm.2013.1235. Epub 2013 Jan 10. Int J Mol Med. 2013. PMID: 23313933 Review. - Manganese Superoxide Dismutase Acetylation and Dysregulation, Due to Loss of SIRT3 Activity, Promote a Luminal B-Like Breast Carcinogenic-Permissive Phenotype.
Zou X, Santa-Maria CA, O'Brien J, Gius D, Zhu Y. Zou X, et al. Antioxid Redox Signal. 2016 Aug 20;25(6):326-36. doi: 10.1089/ars.2016.6641. Epub 2016 Apr 15. Antioxid Redox Signal. 2016. PMID: 26935174 Free PMC article. Review.
Cited by
- Targeting Akt in cancer for precision therapy.
Hua H, Zhang H, Chen J, Wang J, Liu J, Jiang Y. Hua H, et al. J Hematol Oncol. 2021 Aug 21;14(1):128. doi: 10.1186/s13045-021-01137-8. J Hematol Oncol. 2021. PMID: 34419139 Free PMC article. Review. - Oxidative Stress and Antioxidants in Uterine Fibroids: Pathophysiology and Clinical Implications.
AlAshqar A, Lulseged B, Mason-Otey A, Liang J, Begum UAM, Afrin S, Borahay MA. AlAshqar A, et al. Antioxidants (Basel). 2023 Mar 26;12(4):807. doi: 10.3390/antiox12040807. Antioxidants (Basel). 2023. PMID: 37107181 Free PMC article. Review. - Manganese Superoxide Dismutase Acetylation and Regulation of Protein Structure in Breast Cancer Biology and Therapy.
Ogle MM, Trevino R Jr, Schell J, Varmazyad M, Horikoshi N, Gius D. Ogle MM, et al. Antioxidants (Basel). 2022 Mar 25;11(4):635. doi: 10.3390/antiox11040635. Antioxidants (Basel). 2022. PMID: 35453320 Free PMC article. Review. - Application of ex-vivo spheroid model system for the analysis of senescence and senolytic phenotypes in uterine leiomyoma.
Xie J, Xu X, Yin P, Li Y, Guo H, Kujawa S, Chakravarti D, Bulun S, Kim JJ, Wei JJ. Xie J, et al. Lab Invest. 2018 Dec;98(12):1575-1587. doi: 10.1038/s41374-018-0117-5. Epub 2018 Sep 11. Lab Invest. 2018. PMID: 30206313 Free PMC article. - NIPSNAP1 directs dual mechanisms to restrain senescence in cancer cells.
Gao E, Sun X, Thorne RF, Zhang XD, Li J, Shao F, Ma J, Wu M. Gao E, et al. J Transl Med. 2023 Jun 20;21(1):401. doi: 10.1186/s12967-023-04232-1. J Transl Med. 2023. PMID: 37340421 Free PMC article.
References
- Hanahan D., Weinberg R. A., The hallmarks of cancer. Cell 100, 57–70 (2000). - PubMed
- Wallach E. E., Vlahos N. F., Uterine myomas: An overview of development, clinical features, and management. Obstet. Gynecol. 104, 393–406 (2004). - PubMed
- Bulun S. E., Uterine fibroids. N. Engl. J. Med. 369, 1344–1355 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials