Mammalian-Specific Central Myelin Protein Opalin Is Redundant for Normal Myelination: Structural and Behavioral Assessments - PubMed (original) (raw)
. 2016 Nov 17;11(11):e0166732.
doi: 10.1371/journal.pone.0166732. eCollection 2016.
Yumi Sato 1, Koujiro Tohyama 2, Takumi Akagi 3, Tamio Furuse 4, Tetsushi Sadakata 1 5, Mika Tanaka 6, Yo Shinoda 1 7, Tsutomu Hashikawa 3, Shigeyoshi Itohara 6, Yoshitake Sano 8, M Said Ghandour 9, Shigeharu Wakana 4, Teiichi Furuichi 1 8
Affiliations
- PMID: 27855200
- PMCID: PMC5113975
- DOI: 10.1371/journal.pone.0166732
Mammalian-Specific Central Myelin Protein Opalin Is Redundant for Normal Myelination: Structural and Behavioral Assessments
Fumio Yoshikawa et al. PLoS One. 2016.
Abstract
Opalin, a central nervous system-specific myelin protein phylogenetically unique to mammals, has been suggested to play a role in mammalian-specific myelin. To elucidate the role of Opalin in mammalian myelin, we disrupted the Opalin gene in mice and analyzed the impacts on myelination and behavior. Opalin-knockout (Opalin-/-) mice were born at a Mendelian ratio and had a normal body shape and weight. Interestingly, Opalin-/- mice had no obvious abnormalities in major myelin protein compositions, expression of oligodendrocyte lineage markers, or domain organization of myelinated axons compared with WT mice (Opalin+/+) mice. Electron microscopic observation of the optic nerves did not reveal obvious differences between Opalin+/+ and Opalin-/- mice in terms of fine structures of paranodal loops, transverse bands, and multi-lamellae of myelinated axons. Moreover, sensory reflex, circadian rhythm, and locomotor activity in the home cage, as well as depression-like behavior, in the Opalin-/- mice were indistinguishable from the Opalin+/+ mice. Nevertheless, a subtle but significant impact on exploratory activity became apparent in Opalin-/- mice exposed to a novel environment. These results suggest that Opalin is not critical for central nervous system myelination or basic sensory and motor activities under conventional breeding conditions, although it might be required for fine-tuning of exploratory behavior.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures
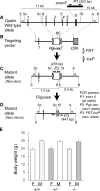
Fig 1. Generation and body weight of Opalin −/− mice.
(A) Genomic structure of mouse Opalin gene (WT allele). Six exons are indicated by thick vertical lines. To evaluate Opalin gene disruption, the exon 6 sequence of the WT allele was amplified by PCR using the internal primer set P1 (shown by arrows), which produced a 203-bp fragment present and absent in the WT and mutant allele, respectively. (B) Construction of targeting vector. A white box and a shaded box indicate the phosphoglycerate kinase 1 (Pgk1) gene promoter-driven neomycin resistance (neo r) gene (Pgk-neo r) and diphtheria toxin A (DTA) cassettes, respectively, flanked by the site-specific FLP recombinase recognition sites FRT (open arrow head). A loxP (shaded arrow head) sequence was present as a vestige of the construction in the right side of the FRT-Pgk-neo-FRT cassette and was not functional. (C) Structure of mutant allele (Neo plus). The region between exon 2 and 6 was deleted and replaced by the _FRT_-Pgk-neo-FRT cassette in the mutant allele. To detect the mutant allele, PCR using the primer set P2 produced a 276-bp fragment in the mutant, but not in the WT. (D) Structure of the neo r cassette-deleted mutant allele (Neo minus). The FRT-Pgk-neo-FRT cassette was deleted by introducing the CAG promoter-driven flippase recombinase gene (CAG-FLP). PCR with the primer set P3 flanking the cassette produced a 441-bp fragment in the Neo minus line. In this study, the Neo plus mouse line was analyzed as a Opalin KO mice, because there were no overt changes between the Neo plus and minus lines. Thin vertical lines show restriction enzyme recognition sites: E, _Eco_RI; Sc, _Sca_I; B, _Bam_HI, Sl, _Sal_I; A, _Ava_I. (E) Body weight (g) of female (F) and male (M) Opalin +/+, Opalin +/− and _Opalin_−/− mice (8 wk). Seven animals for each sex and genotype were used. Error bars = SEM. WT = wildtype; KO = knockout.
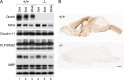
Fig 2. Contents of major myelin proteins are unchanged in Opalin -/- mouse brains, except for Opalin loss, at weaning, young adult, and aged stages compared with WT mice.
(A) Western blot analysis of myelin fractions from Opalin +/+ (lanes 1–3) and _Opalin_−/− (lanes 4–6) mouse brains at 3 wk (weaning stage: lanes 1 and 4), 8 wk (young adult stage: lanes 2 and 5), and 26 wk (adult stage: lanes 3 and 6) using anti-Opalin, anti-MAG, anti-Claudin-11, anti-PLP/DM20, and anti-MBP antibodies. Opalin immunoreactivity is not detectable in _Opalin_−/− samples as expected (top panel, lanes 4–6), although expression of the other tested myelin components is similar to the WT littermates. (B) Immunohistochemical analysis of Opalin +/+ and _Opalin_−/− mouse brains at 16 wk using anti-Opalin antibody. Opalin +/+ mice show intense Opalin immunoreactivity. However, _Opalin_−/− mice show no immunoreactivity for Opalin across various brain regions.
Fig 3. Distribution of myelin-rich regions is unaffected in Opalin −/− mouse brains at young adult and aged stages compared with WT mice.
Immunohistochemical analysis of Opalin +/+ (A, B, E–G) and _Opalin_−/− (C, D, H–J) mouse brains at 16 wk (A, C) and 52 wk (B, D, E–J) using anti-MBP antibody. Immunostaining patterns for MBP at 16 wk and 52 wk do not show any differences between Opalin +/+ and _Opalin_−/− mouse brains. Panels A–D, F, G, I, and J are sagittal sections. Panels E and H are coronal sections. Anterior commissure (ac), cerebellar cortex (Cb), cerebellar white matter (cbwm), corpus callosum (cc), caudate-putamen (CP), corticospinal tract (ct), cerebral cortex (Cx), hippocampal fimbria (fi), hippocampus (Hi), medullary atria of thalamus (ms), myelin basic protein (MBP), optic chiasm (oc), and wild type (WT). Scale bars, 1 mm.
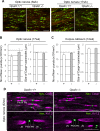
Fig 4. Polarized domain organization of myelinated nerves is not altered in Opalin -/- mice compared with WT mice.
(A) Optic nerves from Opalin +/+ and _Opalin_−/− mice at 8 wk and 17 wk were immunostained with anti-Nav (red) and anti-Caspr (green) antibody. (B) Optic nerves of Opalin +/+ (+) (n = 3) and _Opalin_−/− (−) (n = 3) mice at 17 wk were statistically analyzed. Left, number of Nav-positive puncta (Node of Ranvier) (per mm2). Right, size of Caspr-positive paranodes (longitudinal length of one side of the paranode) (in μm). Data are represented as mean ± SEM. (C) Corpus callosum of Opalin +/+ (+) (n = 5) and _Opalin_−/− (−) (n = 4) mice at 12 wk were statistically analyzed. Left, number of Nav-positive puncta (per mm2). Right, size of Caspr-positive paranodes (in μm). Data are represented as mean ± SEM. (D) Myelin compartments in optic nerves from Opalin +/+ and _Opalin_−/− mice at 52 wk were analyzed by co-immunostaining with anti-pan-Na2+ channel (Nav, marker for the Node of Ranvier [N]) and either anti-Caspr (marker for paranodes [PN]) or anti-K+ channel (Kv1.2, marker for juxtaparanodes [JN]) antibodies. Scale bars, 10 μm in both the top and bottom rows in A, 5 μm in the top row (for Nav and Caspr) in D, 10 μm in the bottom row (for Nav and Kv1.2) in D.
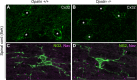
Fig 5. Immunostaining patterns for oligodendrocyte-astrocyte interactions and oligodendrocyte progenitor cells in spinal cords are unchanged in Opalin −/− mice at 8 wk compared with WT mice.
Immunostaining of spinal cords from Opalin +/+ and _Opalin_−/− mice at 8 wk with anti-connexin 32 (Cx32, a component of gap junctions between oligodendrocytes and astrocytes and between paranodal loops) antibody (A and B) or with anti-Nav and anti-NG2 (marker for oligodendrocyte progenitor cells) antibodies (C and D). The immunostaining patterns of these markers shows no differences between Opalin +/+ and _Opalin_−/− mice. Asterisks in A and B represent oligodendrocyte soma. Scale bars, 10 μm. WT = wild type.
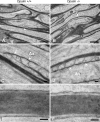
Fig 6. Electron photomicrographs of myelinated optic nerve axons of Opalin +/+ and Opalin −/− mice.
Each optic nerve axon from the Opalin +/+ and _Opalin_−/− mice is firmly wrapped in a myelin sheath (A and B) with well-developed paranodal structures, such as loops, cytoplasmic swellings, and electron-dense transverse bands (arrowheads) juxtaposed to the axolemma (arrows in A and B, and enlarged in C and D). Periodical structures represented by the major dense and intraperiod lines of the myelin sheaths also appear to be similar between the Opalin +/+ and _Opalin_−/− mice. Because the intervals between the paranodal loops subtly varied between samples, probably caused by slight differences in preparation, it was difficult to identify any change in this ultrastructure in the present study. Ax, axoplasm. Scale bars: 1 μm for A and B, and 0.1 μm for C through F.
Fig 7. Behavioral phenotypes of Opalin -/- mice.
(A) Latency to lick the hind paws in the hot plate test (15 weeks old, n = 7/group). (B) Latency to flick the tail in response to heating (17 weeks old, n = 7/group). (C) Locomotor activity of Opalin +/+ (+/+; black square) and _Opalin_−/− (−/−; gray circle) mice in home cage (10–11 weeks old, n = 7/group). Locomotor activities are shown by an arbitrary unit, one for each hour. White and black bars indicate day (light) and night (dark) periods, respectively. (D–F) Exploratory activity in a novel open field (9 weeks old, n = 7/group). (D) Total distance traveled for 20 min. (E) Locomotor activity each for 5 minutes. (F) Ratio of time spent in the center area. (G) Anxiety-like behavior in the light-dark box test (8 weeks old, n = 7/group). Ratio of time spent in the light arena. (H) Depression-like behavior in the tail-suspension test. Percentage of immobility time for 6 min (14 weeks old, n = 7/group). Data are represented as mean ± SEM. a.u., arbitrary unit; *p < 0.05. (Opalin +/+, white column; _Opalin_−/− mice, gray column).
Similar articles
- Opalin, a transmembrane sialylglycoprotein located in the central nervous system myelin paranodal loop membrane.
Yoshikawa F, Sato Y, Tohyama K, Akagi T, Hashikawa T, Nagakura-Takagi Y, Sekine Y, Morita N, Baba H, Suzuki Y, Sugano S, Sato A, Furuichi T. Yoshikawa F, et al. J Biol Chem. 2008 Jul 25;283(30):20830-40. doi: 10.1074/jbc.M801314200. Epub 2008 May 19. J Biol Chem. 2008. PMID: 18490449 Free PMC article. - Age-dependent redistribution and hypersialylation of the central myelin paranodal loop membrane protein Opalin in the mouse brain.
Sato Y, Yoshikawa F, Sadakata T, Shinoda Y, Koebis M, Furuichi T. Sato Y, et al. Neurosci Lett. 2014 Oct 3;581:14-9. doi: 10.1016/j.neulet.2014.08.017. Epub 2014 Aug 19. Neurosci Lett. 2014. PMID: 25153515 - The raft-associated protein MAL is required for maintenance of proper axon--glia interactions in the central nervous system.
Schaeren-Wiemers N, Bonnet A, Erb M, Erne B, Bartsch U, Kern F, Mantei N, Sherman D, Suter U. Schaeren-Wiemers N, et al. J Cell Biol. 2004 Aug 30;166(5):731-42. doi: 10.1083/jcb.200406092. J Cell Biol. 2004. PMID: 15337780 Free PMC article. - Myelin under construction -- teamwork required.
Boiko T, Winckler B. Boiko T, et al. J Cell Biol. 2006 Mar 13;172(6):799-801. doi: 10.1083/jcb.200602101. J Cell Biol. 2006. PMID: 16533942 Free PMC article. Review. - Axon myelination. Myelination without myelin-associated glycoprotein.
Meyer-Franke A, Barres B. Meyer-Franke A, et al. Curr Biol. 1994 Sep 1;4(9):847-50. doi: 10.1016/s0960-9822(00)00190-1. Curr Biol. 1994. PMID: 7529638 Review.
Cited by
- OPALIN is an LGI1 receptor promoting oligodendrocyte differentiation.
Teng XY, Hu P, Zhang CM, Zhang QX, Yang G, Zang YY, Liu ZX, Chen G, Shi YS. Teng XY, et al. Proc Natl Acad Sci U S A. 2024 Aug 6;121(32):e2403652121. doi: 10.1073/pnas.2403652121. Epub 2024 Jul 31. Proc Natl Acad Sci U S A. 2024. PMID: 39083419 Free PMC article. - Microglial activation in an amyotrophic lateral sclerosis-like model caused by Ranbp2 loss and nucleocytoplasmic transport impairment in retinal ganglion neurons.
Cho KI, Yoon D, Yu M, Peachey NS, Ferreira PA. Cho KI, et al. Cell Mol Life Sci. 2019 Sep;76(17):3407-3432. doi: 10.1007/s00018-019-03078-5. Epub 2019 Apr 3. Cell Mol Life Sci. 2019. PMID: 30944974 Free PMC article. - TMEM10 Promotes Oligodendrocyte Differentiation and is Expressed by Oligodendrocytes in Human Remyelinating Multiple Sclerosis Plaques.
de Faria O Jr, Dhaunchak AS, Kamen Y, Roth AD, Kuhlmann T, Colman DR, Kennedy TE. de Faria O Jr, et al. Sci Rep. 2019 Mar 5;9(1):3606. doi: 10.1038/s41598-019-40342-x. Sci Rep. 2019. PMID: 30837646 Free PMC article. - MKK7 deficiency in mature neurons impairs parental behavior in mice.
Shin T, Hiraoka Y, Yamasaki T, Marth JD, Penninger JM, Kanai-Azuma M, Tanaka K, Kofuji S, Nishina H. Shin T, et al. Genes Cells. 2021 Jan;26(1):5-17. doi: 10.1111/gtc.12816. Epub 2020 Nov 18. Genes Cells. 2021. PMID: 33098150 Free PMC article. - Zinc-Finger Protein ZFP488 Regulates the Timing of Oligodendrocyte Myelination and Remyelination.
Cui S, Chen T, Xin D, Chen F, Zhong X, Dong C, Chen X, Chen H, Zhou W, Lin Y, Lu QR. Cui S, et al. J Neurosci. 2024 Sep 25;44(39):e0141242024. doi: 10.1523/JNEUROSCI.0141-24.2024. J Neurosci. 2024. PMID: 39151953 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases