Drosophila ezoana uses an hour-glass or highly damped circadian clock for measuring night length and inducing diapause - PubMed (original) (raw)
Drosophila ezoana uses an hour-glass or highly damped circadian clock for measuring night length and inducing diapause
Koustubh M Vaze et al. Physiol Entomol. 2016 Dec.
Abstract
Insects inhabiting the temperate zones measure seasonal changes in day or night length to enter the overwintering diapause. Diapause induction occurs after the duration of the night exceeds a critical night length (CNL). Our understanding of the time measurement mechanisms is continuously evolving subsequent to Bünning's proposal that circadian systems play the clock role in photoperiodic time measurement (Bünning, 1936). Initially, the photoperiodic clocks were considered to be either based on circadian oscillators or on simple hour-glasses, depending on 'positive' or 'negative' responses in Nanda-Hamner and Bünsow experiments (Nanda & Hammer, 1958; Bünsow, 1960). However, there are also species whose responses can be regarded as neither 'positive', nor as 'negative', such as the Northern Drosophila species Drosophila ezoana, which is investigated in the present study. In addition, modelling efforts show that the 'positive' and 'negative' Nanda-Hamner responses can also be provoked by circadian oscillators that are damped to different degrees: animals with highly sustained circadian clocks will respond 'positive' and those with heavily damped circadian clocks will respond 'negative'. In the present study, an experimental assay is proposed that characterizes the photoperiodic oscillators by determining the effects of non-24-h light/dark cycles (T-cycles) on critical night length. It is predicted that there is (i) a change in the critical night length as a function of T-cycle period in sustained-oscillator-based clocks and (ii) a fixed night-length measurement (i.e. no change in critical night length) in damped-oscillator-based clocks. Drosophila ezoana flies show a critical night length of approximately 7 h irrespective of T-cycle period, suggesting a damped-oscillator-based photoperiodic clock. The conclusion is strengthened by activity recordings revealing that the activity rhythm of D. ezoana flies also dampens in constant darkness.
Keywords: Circadian clock; Drosophila; Nanda–Hamner; damped‐oscillator‐model of photoperiodic clock; diapause; hour‐glass; photoperiodism.
Figures
Figure 1
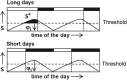
The external coincidence model (Pittendrigh & Minis, 1964). The external coincidence model assumes a circadian rhythm in the abundance of a hypothetical substrate (S), which is converted into its active form S* by a light‐dependent enzymatic reaction. The S level (and hence S*) crosses a threshold and reaches its maximum at a certain time of the day, which is termed as the photo‐inducible phase (ϕi). The coincidence of ϕi with light under long days generates a sufficient amount of S* leading to an induction of long‐day response such as reproduction. In other words, the photo‐inducible phase (ϕi) lies in the light phase. Under short days, ϕi lies in darkness, which leads to short‐day responses such as diapause.
Figure 2
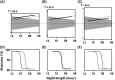
Expected change in the photo‐inducible phase (ϕi) with photoperiod and Zeitgeber period (T). (A)–(C) Qualitative trends in the phase of ϕi as a function of night length at three Zeitgeber periods T < 24 h, T = 24 h and T > 24 h. (D)–(F) Corresponding change in the critical night lengths (
CNL
). For each Zeitgeber periodicity, the night is depicted as a grey area, with night length becoming shorter from left to right (starting with 12 h and ending with 6 h). The phase of ϕi and the photoperiodic response curves of the sustained oscillator based clock are depicted by a thick black line, whereas those of the damped oscillator based clock are indicated by a dashed line. For example, at T = 24 h (B), the transition of ϕi (thick black line) from dark to light occurs at a night length of approximately 9 h and thus has a
CNL
of approximately 9 h (E). On the other hand, the delayed occurrence of ϕi at T < 24h (thick line is pushed up) would make the
CNL
longer (e.g. 10 h) (
A, D
), whereas the earlier occurrence of ϕi (thick line is pushed down) would result in a shorter
CNL
at T > 24h (e.g. 8 h) (
C, F
). The ϕi of the damped oscillator based clock (dashed line) will not show such a dependency on T, thus leading to a constant
CNL
of 9 h at all the Ts. In conclusion, the photoperiodic response curves under T‐cycles can indicate whether the photoperiodic clock behaves as sustained circadian oscillator or a damped circadian oscillator.
Figure 3
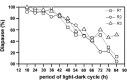
Diapause incidence of
Drosophila ezoana
in response to Nanda–Hamner light regimes. Percentage of diapausing flies plotted as function of period of the light/dark (
LD)
cycle. Each
LD
cycle consists of a fixed light phase of 10 h and a dark phase to make the required periodicity. Three distinct line‐types and symbols represent three separate experiments (runs R1–R3).
Figure 4
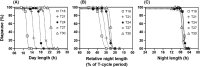
Diapause incidence of
Drosophila ezoana
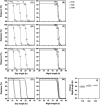
in response to different photoperiods under T18, T21, T24, T27 and T30 (Experiment 1). The photoperiod was varied from 50% to 85% of T‐cycle period (Table 1) and diapause incidence was estimated for each photoperiod in each of the T‐cycles. (A) Percentage of diapausing flies plotted as function of absolute day length. (B) Percentage of diapausing flies plotted as function of relative night length (as percentage of T‐cycle period). (C) Percentage of diapausing flies plotted as function of absolute night length. In each plot, data points for the different T‐cycles are represented by different symbols. Dashed lines depict the nonlinear regression model fitted to the data.
Figure 5
Diapause incidence in
Drosophila ezoana
in response to different day and night lengths under T18, T24 and T30 (Experiment 2). Night length was varied from 5 to 10 h in each of the T‐cycles (Table 2). Percentage of diapausing flies is plotted as function of absolute day length (
A–C
) and of absolute night length (
E–G
) in three separate experiments. Labelling as in Fig. 4. (
D and H
) Regression lines from all the three experiments plotted together demonstrate the differences in the transition from diapause to reproduction across T‐cycles with respect to different day lengths but an almost identical critical night length (
CNL
) at all Zeitgeber periods (T). (I)
CNL
as a function of T. The
CNL
was calculated as 50% in diapause from the regression lines shown in (
H)
(R language package ‘dose–response curve’; Ritz & Streibig, 2005; R Development Core Team, 2008).
Figure 6
Representative individual actograms (
A–E
) and an average actogram (F) of
Drosophila ezoana
in constant darkness (
DD
). The average actogram (D) is calculated out of all 64 recorded flies. The flies were transferred from constant light (
LL
) to
DD
as indicated by the black arrow. The first onset of activity is indicated by a white arrow. In the average actogram, a grey dotted line indicates the end of activity during the few first days in
DD
.
Figure 7
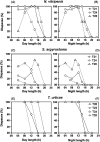
Diapause incidence in response to different day and night lengths under different T‐cycles in
Nasonia vitripenis
,
Sarcophaga argyrostoma
and
Tetranychus urticae
. The data reported were extracted from old Nanda–Hamner studies in the parasitic wasp
N. vitripenis
(Saunders, 1974), the flesh fly
S. argyrostoma
(Saunders, 1973) and the spider mite
T. urticae
(Vaz Nunes & Veerman, 1986). These studies conducted Nanda–Hamner experiments using a range of photoperiod conditions, thus allowing us to extract diapause incidence for more than one combination of photoperiods and night lengths for each
LD
periodicity. The extracted diapause incidence data were then plotted as a function of day length (
A, C, E
) and night length (
B, D, F
). Distinct line‐types and symbols in each plot represent different T‐cycle periods.
Similar articles
- Nanda-Hamner Curves Show Huge Latitudinal Variation but No Circadian Components in Drosophila Montana Photoperiodism.
Lankinen P, Kastally C, Hoikkala A. Lankinen P, et al. J Biol Rhythms. 2021 Jun;36(3):226-238. doi: 10.1177/0748730421997265. Epub 2021 Mar 22. J Biol Rhythms. 2021. PMID: 33745359 Free PMC article. - Drosophila ezoana uses morning and evening oscillators to adjust its rhythmic activity to different daylengths but only the morning oscillator to measure night length for photoperiodic responses.
Vaze KM, Manoli G, Helfrich-Förster C. Vaze KM, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):535-548. doi: 10.1007/s00359-023-01646-6. Epub 2023 Jun 17. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37329349 Free PMC article. - Evolutionary links between circadian clocks and photoperiodic diapause in insects.
Meuti ME, Denlinger DL. Meuti ME, et al. Integr Comp Biol. 2013 Jul;53(1):131-43. doi: 10.1093/icb/ict023. Epub 2013 Apr 24. Integr Comp Biol. 2013. PMID: 23615363 Free PMC article. Review. - Deciphering time measurement: the role of circadian 'clock' genes and formal experimentation in insect photoperiodism.
Saunders DS, Bertossa RC. Saunders DS, et al. J Insect Physiol. 2011 May;57(5):557-66. doi: 10.1016/j.jinsphys.2011.01.013. Epub 2011 Feb 2. J Insect Physiol. 2011. PMID: 21295039 Review.
Cited by
- Characterization of pre-diapause phase in the northern Drosophila species D. ezoana.
Vaze KM, Manoli G, Helfrich-Förster C. Vaze KM, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Nov;210(6):901-908. doi: 10.1007/s00359-024-01707-4. Epub 2024 Jun 25. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 38916659 - A clock for all seasons.
Helfrich-Förster C, Rieger D. Helfrich-Förster C, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):473-480. doi: 10.1007/s00359-024-01711-8. Epub 2024 Jun 19. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 38896260 Free PMC article. - Variation in photoperiod response corresponds to differences in circadian light sensitivity in northern and southern Nasonia vitripennis lines.
Floessner TSE, Benetta ED, Beersma DGM, Hut RA. Floessner TSE, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):667-676. doi: 10.1007/s00359-023-01674-2. Epub 2023 Oct 18. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37853248 Free PMC article. - Time measurement in insect photoperiodism: external and internal coincidence.
Saunders DS. Saunders DS. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):513-525. doi: 10.1007/s00359-023-01648-4. Epub 2023 Sep 12. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37697123 Free PMC article. Review. - The seasons within: a theoretical perspective on photoperiodic entrainment and encoding.
Schmal C. Schmal C. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):549-564. doi: 10.1007/s00359-023-01669-z. Epub 2023 Sep 2. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37659985 Free PMC article. Review.
References
- Bradshaw, W.E. & Holzapfel, C.M. (2007) Evolution of animal photoperiodism. Annual Review of Ecology, Evolution, and Systematics, 38, 1–25.
- Bradshaw, W.E. & Lounibos, L.P. (1972) Photoperiodic control of development in the pitcher‐plant mosquito, Wyeomyia smithii . Canadian Journal of Zoology, 50, 713–719.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases