FGF14 is a regulator of KCNQ2/3 channels - PubMed (original) (raw)
FGF14 is a regulator of KCNQ2/3 channels
Juan Lorenzo Pablo et al. Proc Natl Acad Sci U S A. 2017.
Abstract
KCNQ2/3 (Kv7.2/7.3) channels and voltage-gated sodium channels (VGSCs) are enriched in the axon initial segment (AIS) where they bind to ankyrin-G and coregulate membrane potential in central nervous system neurons. The molecular mechanisms supporting coordinated regulation of KCNQ and VGSCs and the cellular mechanisms governing KCNQ trafficking to the AIS are incompletely understood. Here, we show that fibroblast growth factor 14 (FGF14), previously described as a VGSC regulator, also affects KCNQ function and localization. FGF14 knockdown leads to a reduction of KCNQ2 in the AIS and a reduction in whole-cell KCNQ currents. FGF14 positively regulates KCNQ2/3 channels in a simplified expression system. FGF14 interacts with KCNQ2 at a site distinct from the FGF14-VGSC interaction surface, thus enabling the bridging of NaV1.6 and KCNQ2. These data implicate FGF14 as an organizer of channel localization in the AIS and provide insight into the coordination of KCNQ and VGSC conductances in the regulation of membrane potential.
Keywords: FGF14; KCNQ2; ankyrin-G; axon initial segment; fibroblast growth factor homologous factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
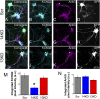
Fig. 1.
FGF14 knockdown leads to loss of AIS-localized KCNQ2. (A_–_D) Neurons transfected with scrambled control shRNA (GFP reporter, white signal) exhibit localization of (B) KCNQ2 to the AIS as marked by (C) ank-G. Nearby untransfected neurons have the same pattern. (E_–_H) Neurons transfected with FGF14-targeted shRNA (GFP reporter, white signal) lose more than half of (F) KCNQ2 immunoreactivity at the (G) AIS. Nearby untransfected neurons are unaffected. (I_–_L) Neurons transfected with FGF13-targeted shRNA do not exhibit loss of (J) KCNQ2 at the (K) AIS. (M) Quantification of KCNQ2 immunoreactivity via integrated pixel intensity reveals that 14KD but not 13KD significantly reduces KCNQ2 localization compared with Scr. (N) Ank-G is not significantly affected across treatments. (*P < 0.05; Scr n = 34, 14KD n = 21, 13KD n = 40).
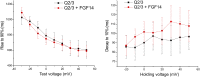
Fig. 2.
FGF14 increases KCNQ2/3 current in a heterologous expression system. (A) Exemplar K+ currents from HEK cells transfected with KCNQ2 and KCNQ3 (Q2/Q3) only or with FGF14 added (Q2/Q3 + FGF14). Currents were elicited via a voltage-step protocol in whole-cell voltage clamp. K+ currents from cells transfected with FGF14 were larger than currents from control cells. (B) Maximum current densities from two different test voltages show that the presence of FGF14 promotes larger KCNQ2/3 currents (*P < 0.05, n = 11, Q2/Q3; n = 14, Q2/Q3 + FGF14). (C) The V1/2 for activation extracted from tail currents (Q2/Q3 = −17.83 ± 2.57 mV, Q2/Q3 + FGF14 = −21.24 ± 2.33 mV; P = 0.34) and slope factor (Q2/Q3 = 9.83 ± 0.46 mV, Q2/Q3 + FGF14 = 9.80 ± 0.74 mV; P = 0.98) were not significantly changed. (D) More KCNQ2 channels are present at the cell membrane when FGF14 is expressed, as revealed by surface biotinylation (representative experiment from three independent trials).
Fig. S1.
(Left) Analysis of time constants of rise from a holding potential of −90 mV to the indicated potential. No significant difference between treatments is observed. (Right) Analysis of time constants of decay from the indicated voltage to −90 mV. No significant difference is observed.
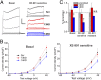
Fig. 3.
FGF14 knockdown leads to a decrease in XE-991–sensitive outward currents. (A) Currents from DIV10–11 neurons recorded in the presence of TTX, Cd2+, and 4-AP were elicited from a holding potential of −90 mV to steps from −100 mV to 40 mV (n, Scr = 16, 14KD = 21, 13KD = 16). KCNQ currents were defined as the noninactivating component of outward potassium current sensitive to 20 μm of XE-991. KCNQ currents from neurons transfected with FGF14-targeted shRNA (14KD) but not FGF13-targeted shRNA (13KD) neurons were smaller than currents from neurons transfected with a scrambled control shRNA (Scr). (Scale bar, 1 nA and 200 ms.) (B) Current–voltage relationships plotted for Scr, 13KD, and 14KD. Shown are basal current densities before application of XE-991 and current densities of the XE-991–sensitive component. (C) Maximum current amplitudes from the last 100 ms of the 20-mV voltage step were significantly lower for 14KD than for Scr or 13KD. A smaller component of this current was XE-991 sensitive in 14KD neurons, indicating a loss of KCNQ currents. XE-991–resistant currents were not significantly changed although a decremental trend was observed (normalized mean ± error propagated SEM, *P < 0.05, ANOVA followed by Fisher’s least significant difference (LSD).
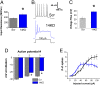
Fig. 4.
FGF14 knockdown changes intrinsic membrane properties and action potential generation. (A) The input resistance of 14KD neurons is higher than for Scr neurons. (B) Examples of action potential trains generated from 100-pA injections into both Scr and 14KD neurons. In 14KD neurons, the rise in interspike voltage during the current injection step is higher. Quantification is shown in C. (D) The threshold for action potentials subsequent to the first action potential of a train is higher in 14KD neurons than it is in Scr control neurons. (E) Increasing current injections lead to decreased numbers of action potentials generated from 14KD neurons compared with Scr neurons. (*P < 0.05, Student’s t test; n = 11–15 cells each).
Fig. 5.
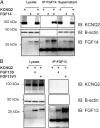
FGF14 but not FGF13 binds to KCNQ2. (A) Coimmunoprecipitation experiments with heterologous expression of KCNQ2 and FGF14 show that immunoprecipitating FGF14 leads to the coimmunoprecipitation of KCNQ2. This result is not true when one of these proteins is omitted. KCNQ2 signal is decreased in input material (supernatant) only when FGF14 is present and immunoprecipitated, again indicating binding (representative experiment from four independent trials). Dark bands below the FGF14 bands are IgG from the antibodies used for the IP. (B) Immunoprecipitation of FGF13 (splice variants FGF13S or FGF13VY) leads to no discernible signal for coimmunoprecipitation of KCNQ2. This finding indicates that FGF13 does not bind to KCNQ2 (representative experiment from three independent trials). Dark bands below the FGF13 bands are IgG from the antibodies used for the IP.
Fig. 6.
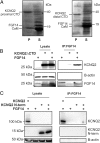
FGF14 binding to KCNQ2 does not require the C terminus nor is the KCNQ2 N terminus sufficient for binding. (A) Copurification experiments with a 6xHis-tagged KCNQ2 C terminus show that calmodulin (CaM) binds, whereas FGF14 does not. A different KCNQ2 C terminus construct, without the CaM binding site, copurifies neither FGF14 nor CaM. (B) Immunoprecipitation of FGF14 is still capable of immunoprecipitating a KCNQ2 construct that lacks a C terminus. Omission of either protein does not yield a significant signal. (C) Immunoprecipitation of FGF14 does not coimmunoprecipitate HA-tagged KCNQ2 N terminus. Coimmunoprecipitation of the full-length HA-tagged KCNQ2 is shown as a positive control.
Fig. 7.
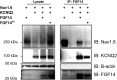
FGF14 binds to KCNQ2 at an interface distinct from NaV binding. Immunoprecipitation of wild-type FGF14 coimmunoprecipitates both NaV1.6 and KCNQ2. In contrast, immunoprecipitation of a NaV binding-deficient FGF14RA mutant does not coimmunoprecipitate NaV1.6, but still retains the capability to coimmunoprecipitate KCNQ2 (representative experiment from three independent trials).
Fig. 8.
FGF14 bridges NaV1.6 and KCNQ2. Immunoprecipitation of KCNQ2 is only capable of coimmunoprecipitating NaV1.6 in the presence of FGF14. Omission of any of these three components leads to no signal (representative experiment from three independent trials).
Similar articles
- An Ankyrin-G N-terminal Gate and Protein Kinase CK2 Dually Regulate Binding of Voltage-gated Sodium and KCNQ2/3 Potassium Channels.
Xu M, Cooper EC. Xu M, et al. J Biol Chem. 2015 Jul 3;290(27):16619-32. doi: 10.1074/jbc.M115.638932. Epub 2015 May 21. J Biol Chem. 2015. PMID: 25998125 Free PMC article. - FGF14 localization and organization of the axon initial segment.
Xiao M, Bosch MK, Nerbonne JM, Ornitz DM. Xiao M, et al. Mol Cell Neurosci. 2013 Sep;56:393-403. doi: 10.1016/j.mcn.2013.07.008. Epub 2013 Jul 26. Mol Cell Neurosci. 2013. PMID: 23891806 Free PMC article. - Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment.
Rasmussen HB, Frøkjaer-Jensen C, Jensen CS, Jensen HS, Jørgensen NK, Misonou H, Trimmer JS, Olesen SP, Schmitt N. Rasmussen HB, et al. J Cell Sci. 2007 Mar 15;120(Pt 6):953-63. doi: 10.1242/jcs.03396. Epub 2007 Feb 20. J Cell Sci. 2007. PMID: 17311847 - Made for "anchorin": Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons.
Cooper EC. Cooper EC. Semin Cell Dev Biol. 2011 Apr;22(2):185-92. doi: 10.1016/j.semcdb.2010.10.001. Epub 2010 Oct 19. Semin Cell Dev Biol. 2011. PMID: 20940059 Free PMC article. Review. - Trafficking mechanisms underlying Nav channel subcellular localization in neurons.
Solé L, Tamkun MM. Solé L, et al. Channels (Austin). 2020 Dec;14(1):1-17. doi: 10.1080/19336950.2019.1700082. Channels (Austin). 2020. PMID: 31841065 Free PMC article. Review.
Cited by
- Divide, multitask, and conquer: Coordination in channel regulation.
Pablo JL, Pitt GS. Pablo JL, et al. Channels (Austin). 2017 Jul 4;11(4):268-270. doi: 10.1080/19336950.2017.1292814. Epub 2017 Mar 2. Channels (Austin). 2017. PMID: 28282251 Free PMC article. No abstract available. - Parvalbumin interneuron impairment causes synaptic transmission deficits and seizures in SCN8A developmental and epileptic encephalopathy.
Miralles RM, Boscia AR, Kittur S, Hanflink JC, Panchal PS, Yorek MS, Deutsch TCJ, Reever CM, Vundela SR, Wengert ER, Patel MK. Miralles RM, et al. JCI Insight. 2024 Oct 22;9(20):e181005. doi: 10.1172/jci.insight.181005. JCI Insight. 2024. PMID: 39435659 Free PMC article. - Fibroblast growth factor signaling in axons: from development to disease.
Tomé D, Dias MS, Correia J, Almeida RD. Tomé D, et al. Cell Commun Signal. 2023 Oct 16;21(1):290. doi: 10.1186/s12964-023-01284-0. Cell Commun Signal. 2023. PMID: 37845690 Free PMC article. Review. - Exploring the Genomic Patterns in Human and Mouse Cerebellums Via Single-Cell Sequencing and Machine Learning Method.
Li Z, Wang D, Liao H, Zhang S, Guo W, Chen L, Lu L, Huang T, Cai YD. Li Z, et al. Front Genet. 2022 Mar 4;13:857851. doi: 10.3389/fgene.2022.857851. eCollection 2022. Front Genet. 2022. PMID: 35309141 Free PMC article. - The IGSF1, Wnt5a, FGF14, and ITPR1 Gene Expression and Prognosis Hallmark of Prostate Cancer.
Ebrahimi S, Rezaei Fakhrnezhad F, Jahangiri S, Borjkhani M, Behboodi R, Monfaredan A. Ebrahimi S, et al. Rep Biochem Mol Biol. 2022 Apr;11(1):44-53. doi: 10.52547/rbmb.11.1.44. Rep Biochem Mol Biol. 2022. PMID: 35765527 Free PMC article.
References
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548–562. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources