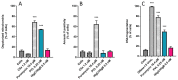Above and beyond C5a Receptor Targeting by Staphylococcal Leucotoxins: Retrograde Transport of Panton-Valentine Leucocidin and γ-Hemolysin - PubMed (original) (raw)
Above and beyond C5a Receptor Targeting by Staphylococcal Leucotoxins: Retrograde Transport of Panton-Valentine Leucocidin and γ-Hemolysin
Gaëlle Zimmermann-Meisse et al. Toxins (Basel). 2017.
Abstract
Various membrane receptors associated with the innate immune response have recently been identified as mediators of the cellular action of Staphylococcus aureus leucotoxins. Two of these, the Panton-Valentine leucotoxin LukS-PV/LukF-PV and the γ-hemolysin HlgC/HlgB, bind the C5a complement-derived peptide receptor. These leucotoxins utilize the receptor to induce intracellular Ca2+ release from internal stores, other than those activated by C5a. The two leucotoxins are internalized with the phosphorylated receptor, but it is unknown whether they divert retrograde transport of the receptor or follow another pathway. Immunolabeling and confocal microscopic techniques were used to analyze the presence of leucotoxins in endosomes, lysosomes, endoplasmic reticulum, and Golgi. The two leucotoxins apparently followed retrograde transport similar to that of the C5a peptide-activated receptor. However, HlgC/HlgB reached the Golgi network very early, whereas LukS-PV/LukF-PV followed slower kinetics. The HlgC/HlgB leucotoxin remained in neutrophils 6 h after a 10-min incubation of the cells in the presence of the toxin with no signs of apoptosis, whereas apoptosis was observed 3 h after neutrophils were incubated with LukS-PV/LukF-PV. Such retrograde transport of leucotoxins provides a novel understanding of the cellular effects initiated by sublytic concentrations of these toxins.
Keywords: C5aR binding leucotoxins; Fura-2 Calcium fluorimetry; Staphylococcus aureus; confocal microscopy; human neutrophils; retrograde transport.
Conflict of interest statement
Conflicts of InterestThe funding agencies had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript. None of the results obtained are part of a patent or commercial product. E.J. is permanent staff of the CNRS (governmental) and G.P. is a permanent faculty member of the Université de Strasbourg. None of the authors has a conflict of interest to declare.
Figures
Figure 1
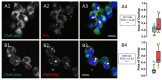
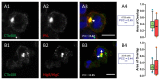
Both PVL and HlgC/HlgB are found with the phosphorylated C5a receptor in intracellular organelles. (A1–A4) Human neutrophils were incubated for 10 min with the PVL (0.25 nM), the toxin was removed, and the neutrophils were maintained at 37 °C for an additional 20 min. The cells were fixed and immunolabeled with: C5aR (A1); and LukS-subunit (A2) antibodies. (A3) A merged image of A1 and A2. (A4) CellProfiler software was used to calculate Pearson’s correlation coefficient (PCC) between the two fluorescent markers. Values are compared with results of control cells, which were processed as experimental cells but in the absence of the leucotoxin. Box-and-Whisker’s plots show the relationship between the fluorescent labels by overlapping the labeled surfaces calculated with CellProfiler software. The green Box and Whiskers (median and percentiles) correspond to the percentage of total C5aR labeled area stained by the anti-leucotoxin antibody; the red Box is the percentage of the total surface labeled by the leucotoxin also stained with the anti-C5aR antibody. The number of cells considered is indicated above the PCC value. Arrows in the merged image indicate the points of most visible overlap between the two antibodies. (B1–B4) Human neutrophils incubated in the presence of 0.5 nM HlgC/HlgB. The results are presented as in (A1–A4) using CellProfiler software. Scale bars, 10 µm.
Figure 2
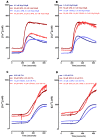
Leucotoxins require optimal buffer conditions for neutrophils to fully develop their activity. Human neutrophils recovered in a RPMI-10% FBS culture medium after purification were incubated for 1 h in 5 µM Fura-2 solution at 37 °C, washed by mild centrifugation and filtration, and maintained in the same buffer in the dark until use. (A) HlgC/HlgB-challenged neutrophils and the effect of blocking the store operated channels using 2-APB under control conditions and after disrupting the lysosomal compartment with GPN. (B) Effect of treating human neutrophils in the presence of YM 58483, which blocks the store operated channels, before challenge with 0.5 nM HlgC/HlgB as in (A). Human neutrophils from the same batches were used to analyze the effect of the PVL after incubation under identical conditions. Results are shown in (C,D). Traces represent the mean of a minimum of three independent experiments. The cells were incubated for 30 min in the presence of drugs (GPN, 26APB, and YM 58483), if needed, before the fluorescence recording. The toxins were added 180 s after starting to record.
Figure 3
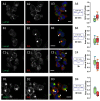
PVL and HlgC/HlgB leucotoxins do not remain in the early endosome (Rab5 labeling), the recycling endosome (Rab11b labeling), or the endoplasmic reticulum (PDI labeling). Examples of human neutrophils incubated with 0.25 nM PVL ((A1,2) 10 min; (C1,2) 30 min; and (E1,2) 30 min) or 0.5 nM HlgC/HlgB ((B1,2) 10 min; (D1,2) 30 min; and (F1,2) 30 min) and stained with antibodies against Rab5 (A1,B1), which concentrates in early endosomes. Labeling with anti-Rab11a antibody (C1,D1) highlights recycling endosomes, whereas the anti-PDI antibody (E1,F1) targets the endoplasmic reticulum. Arrows in each image indicate segregation between leucotoxin labeling and the three cell compartments. Overlap between the two markers can be observed in some cases, although the PCC values (A2–F2) for fluorescence co-distribution were low and not significantly different from control values, suggesting a random distribution. As in Figure 1, the Box-and-Whiskers plots (median and percentiles) are used to show the relationship between the fluorescent labels through overlap of the labeled surfaces. Green boxes indicate the values for the fraction of total surface labeled by: the anti-RAB5 antibody (A2,B2); the anti-RAB11A antibody (C2,D2); and the anti-PDI antibody (E2,F2) that was also labeled by the anti-leucotoxin antibody. Red boxes represent the percentage of total area labeled by the anti-leucotoxin antibody and stained by antibodies against the specific cellular compartments. The numbers of cells considered are indicated above the respective PCC values. In all cases, the percentage of surface labeled is compared with that of a control where the cells were processed with the same antibodies, but in the absence of leucotoxin. Scale bars, 10 µm.
Figure 4
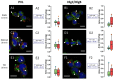
The PVL reaches the trans-Golgi network (TGN) 30 min after transiting through the lysosomal compartment. The human neutrophil lysosomal compartment was incubated with 0.25 nM PVL for: 20 (A1–A4); 40 (B1–B4); and 180 min (C1–C4) and immunostained with the anti-LAMP1 antibody. A significant proportion of the total surface labeled with the antibody is also associated with PVL-related fluorescence (arrows). Labeling was mainly concentrated in the area proximal to the nuclei. (B1–B3) The results after 40 min. (D1–D3) The TGN labeled with the anti-M6PR antibody after a 40 min incubation in the presence of the PVL. The Box-and-Whiskers plot shows the overlapping surfaces labeled by the two antibodies compared to the control. Red boxes show the percentage of total area labeled by the anti-leucotoxin antibody that is also stained by the other antibody. The number of cells considered in each case and the PCC for specific labeling are indicated in insets from (A4–D4). Scale bars, 10 µm.
Figure 5
The HlgC/HlgB begins concentrating in the trans-Golgi network (TGN) 10 min after binding to the receptor. Human neutrophils incubated with 0.5 nM HlgC/HlgB for: 10 (A1–A4); 20 (B1–B4); and 180 min (C1–C4) were immunostained with the anti-M6PR antibody to highlight the TGN. Cells were processed as described in Figure 4. The results indicate that HlgC/HlgB began concentrating in the TGN after 10 min (A1–A4). A significant proportion of fluorescence emitted by the labels overlapped with the others, as shown in the Box-and-Whiskers plots. (D1–D4) An example of the segregation systematically observed after 40 min between the lysosomal compartment (stained by the anti-LAMP1 antibody) and intracellular localization of HlgC/HlgB. Scale bars, 10 µm.
Figure 6
Co-localization of leucotoxins with the Cholera toxin b-subunit in the trans-Golgi network (TGN). Examples of human neutrophils incubated in the presence of: 0.25 nM PVL (A1–A4); or 0.5 nM HlgC/HlgB (B1–B4) for 40 min and then counterstained with the Alexa-488-derived b-subunit of the Cholera toxin, which binds GM1 gangliosides found in lipid rafts and subsequently concentrates in the TGN. (A4,B4) The overlapping percentage of surface labeled through Box-and-Whiskers plots. Scale bars, 10 µm.
Figure 7
Human neutrophils overcame the intracellular presence of HlgC/HlgB for more than 6 h, whereas the PVL initiated apoptosis during this period. Three independent tests were used to estimate the initiation of apoptosis in human neutrophils incubated for 3 h in the presence of 0.25 nM PVL or for 6 h with 0.5 nM HlgC/HlgB by flow cytometry. (A) CCCP fluorescence associated with depolarized mitochondria showing 54% ± 1% of the PVL-treated cells compared to none of the HlgC/HlgB with labeling above background. (B) Annexin V labeling of externalized phosphatidylserine failed to highlight apoptotic human neutrophils treated with the PVL, whereas the TUNEL assay (C) confirmed that approximately 50% of PVL-treated cells were apoptotic. All three apoptosis detection protocols failed to reveal apoptotic activity in human neutrophils in the presence of HlgC/HlgB during the same time period.
Similar articles
- Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding.
Meyer F, Girardot R, Piémont Y, Prévost G, Colin DA. Meyer F, et al. Infect Immun. 2009 Jan;77(1):266-73. doi: 10.1128/IAI.00402-08. Epub 2008 Oct 6. Infect Immun. 2009. PMID: 18838523 Free PMC article. - Internalization of staphylococcal leukotoxins that bind and divert the C5a receptor is required for intracellular Ca(2+) mobilization by human neutrophils.
Tawk MY, Zimmermann K, Bossu JL, Potrich C, Bourcier T, Dalla Serra M, Poulain B, Prévost G, Jover E. Tawk MY, et al. Cell Microbiol. 2015 Aug;17(8):1241-57. doi: 10.1111/cmi.12434. Epub 2015 Mar 30. Cell Microbiol. 2015. PMID: 25737084 - Channel-forming leucotoxins from Staphylococcus aureus cause severe inflammatory reactions in a rabbit eye model.
Siqueira JA, Speeg-Schatz C, Freitas FI, Sahel J, Monteil H, Prévost G. Siqueira JA, et al. J Med Microbiol. 1997 Jun;46(6):486-94. doi: 10.1099/00222615-46-6-486. J Med Microbiol. 1997. PMID: 9350201 - Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes.
Kaneko J, Kamio Y. Kaneko J, et al. Biosci Biotechnol Biochem. 2004 May;68(5):981-1003. doi: 10.1271/bbb.68.981. Biosci Biotechnol Biochem. 2004. PMID: 15170101 Review. - Leukocidal toxins of staphylococci.
Szmigielski S, Prévost G, Monteil H, Colin DA, Jeljaszewicz J. Szmigielski S, et al. Zentralbl Bakteriol. 1999 Apr;289(2):185-201. doi: 10.1016/s0934-8840(99)80105-4. Zentralbl Bakteriol. 1999. PMID: 10360319 Review.
Cited by
- Prevalence and characteristics of Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) isolated from chicken meat in the province of Quebec, Canada.
Bernier-Lachance J, Arsenault J, Usongo V, Parent É, Labrie J, Jacques M, Malouin F, Archambault M. Bernier-Lachance J, et al. PLoS One. 2020 Jan 10;15(1):e0227183. doi: 10.1371/journal.pone.0227183. eCollection 2020. PLoS One. 2020. PMID: 31923238 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous