Superoxide dismutase/catalase mimetic EUK-134 prevents diaphragm muscle weakness in monocrotalin-induced pulmonary hypertension - PubMed (original) (raw)
Superoxide dismutase/catalase mimetic EUK-134 prevents diaphragm muscle weakness in monocrotalin-induced pulmonary hypertension
Koichi Himori et al. PLoS One. 2017.
Abstract
Patients with pulmonary hypertension (PH) suffer from inspiratory insufficiency, which has been associated with intrinsic contractile dysfunction in diaphragm muscle. Here, we examined the role of redox stress in PH-induced diaphragm weakness by using the novel antioxidant, EUK-134. Male Wistar rats were randomly divided into control (CNT), CNT + EUK-134 (CNT + EUK), monocrotaline-induced PH (PH), and PH + EUK groups. PH was induced by a single intraperitoneal injection of monocrotaline (60 mg/kg body weight). EUK-134 (3 mg/kg body weight/day), a cell permeable mimetic of superoxide dismutase (SOD) and catalase, was daily intraperitoneally administered starting one day after induction of PH. After four weeks, diaphragm muscles were excised for mechanical and biochemical analyses. There was a decrease in specific tetanic force in diaphragm bundles from the PH group, which was accompanied by increases in: protein expression of NADPH oxidase 2/gp91phox, SOD2, and catalase; 3-nitrotyrosine content and aggregation of actin; glutathione oxidation. Treatment with EUK-134 prevented the force decrease and the actin modifications in PH diaphragm bundles. These data show that redox stress plays a pivotal role in PH-induced diaphragm weakness. Thus, antioxidant treatment can be a promising strategy for PH patients with inspiratory failure.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
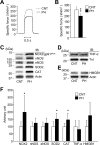
Fig 1. Specific force depression is accompanied by upregulation of redox enzymes in diaphragm from PH rat.
A: typical examples (100 Hz stimulation frequency, 600 ms train duration) of specific force in diaphragm fiber bundles from CNT and PH rats. B: Specific force at 100 Hz stimulation frequency. _C_-E: representative western blots illustrating the levels of NADPH oxidase (NOX2/gp91phox), neuronal nitric oxide synthase (nNOS), endothelial NOS (eNOS), superoxide dismutase 2 (SOD2), catalase (CAT), tumor necrosis factor (TNF)-α and high mobility group box 1 (HMGB1). Inducible NOS (iNOS) was not detected in either group. F: quantification of the levels of redox-related proteins and inflammatory mediators normalized to actin or troponin I (TnI) content. Data show mean ± SD for 5–8 rats in each group. *P < 0.05, **P < 0.01 vs. CNT.
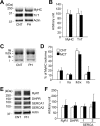
Fig 2. Neither contractile proteins nor excitation-contraction coupling proteins are altered in PH diaphragm muscles.
A: Stain free images of myosin heavy chain (MyHC) and western blots of troponin (Tn) T. B: the expression levels of MyHC or TnT normalized to actin content. C: electrophoretically separated MyHC isoforms. D: percentage distribution of MyHC isoforms: I, slow myosin isoform; IIa, IId/x, and IIb, fast myosin isoforms. E: representative western blots of ryanodine receptor (RyR1), dihydropyridine receptor α2 subunit (DHPR), sarcoplasmic reticulum Ca2+-ATPase (SERCA) 1, and SERCA2. F: the expression levels of RyR1, DHPR, SERCA1, or SERCA2 normalized to actin content. Control (CNT), white bars; AIA, black bars. Data represent mean ± SD for 4–8 rats in each group.
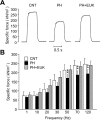
Fig 3. Antioxidant treatment prevents contractile dysfunction in PH diaphragm muscles.
A: typical examples (120 Hz stimulation frequency, 600 ms train duration) of specific force in diaphragm fiber bundles from CNT and PH rats with or without EUK-134 (EUK) treatment. B: specific force-frequency relationship. Data show mean ± SD for 7–10 rats in each group. **P < 0.01 vs. CNT.
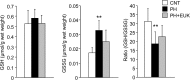
Fig 4. GSH: GSSG ratio is decreased in diaphragm muscles from PH rat.
The cytoplasm levels of GSH, GSSG, and the GSH: GSSG ratio in diaphragm muscles from CNT and PH rats with or without EUK-134 (EUK) treatment. Data show mean ± SD for 5–8 rats in each group. **P < 0.01 vs. CNT.
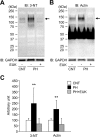
Fig 5. 3-nitrotyrosine content is increased in actin aggregates from PH diaphragm muscles.
A and B: representative western blots for 3-nitrotyrosine (3-NT) and actin in diaphragm muscles from CNT and PH rats with or without treatment with EUK-134 (EUK). C: intensities for the protein band at ~130 kDa (indicated by arrows) in 3-NT and actin were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) content. Data show mean ± SD for 6 rats in each group. **P < 0.01 vs. CNT.
Similar articles
- Protective effects of a superoxide dismutase/catalase mimetic compound against paraquat pneumotoxicity in rat lung.
Shopova VL, Dancheva VY, Salovsky PT, Stoyanova AM. Shopova VL, et al. Respirology. 2009 May;14(4):504-10. doi: 10.1111/j.1440-1843.2009.01531.x. Respirology. 2009. PMID: 19645869 - Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress.
Zhang HJ, Doctrow SR, Xu L, Oberley LW, Beecher B, Morrison J, Oberley TD, Kregel KC. Zhang HJ, et al. FASEB J. 2004 Oct;18(13):1547-9. doi: 10.1096/fj.04-1629fje. Epub 2004 Aug 19. FASEB J. 2004. PMID: 15319374 - The manganese-salen compound EUK-134 and N-acetyl cysteine rescue from zinc- and paraquat-induced toxicity in rat polymorphonuclear leukocytes.
Kumar A, Shukla S, Chauhan AK, Singh D, Pandey HP, Singh C. Kumar A, et al. Chem Biol Interact. 2015 Apr 25;231:18-26. doi: 10.1016/j.cbi.2015.02.012. Epub 2015 Feb 24. Chem Biol Interact. 2015. PMID: 25724285 - Salen Mn complexes mitigate radiation injury in normal tissues.
Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, Medhora M, Molthen R, Moulder JE, Sonis ST, Tofilon PJ, Doctrow SR. Rosenthal RA, et al. Anticancer Agents Med Chem. 2011 May 1;11(4):359-72. doi: 10.2174/187152011795677490. Anticancer Agents Med Chem. 2011. PMID: 21453241 Free PMC article. Review. - Diaphragm plasticity in aging and disease: therapies for muscle weakness go from strength to strength.
Greising SM, Ottenheijm CAC, O'Halloran KD, Barreiro E. Greising SM, et al. J Appl Physiol (1985). 2018 Aug 1;125(2):243-253. doi: 10.1152/japplphysiol.01059.2017. Epub 2018 Apr 19. J Appl Physiol (1985). 2018. PMID: 29672230 Free PMC article. Review.
Cited by
- Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes.
Rouco L, González-Noya AM, Pedrido R, Maneiro M. Rouco L, et al. Antioxidants (Basel). 2020 Aug 10;9(8):727. doi: 10.3390/antiox9080727. Antioxidants (Basel). 2020. PMID: 32785017 Free PMC article. Review. - Skeletal and Respiratory Muscle Dysfunctions in Pulmonary Arterial Hypertension.
Riou M, Pizzimenti M, Enache I, Charloux A, Canuet M, Andres E, Talha S, Meyer A, Geny B. Riou M, et al. J Clin Med. 2020 Feb 3;9(2):410. doi: 10.3390/jcm9020410. J Clin Med. 2020. PMID: 32028638 Free PMC article. Review. - Targeting oxidative stress in disease: promise and limitations of antioxidant therapy.
Forman HJ, Zhang H. Forman HJ, et al. Nat Rev Drug Discov. 2021 Sep;20(9):689-709. doi: 10.1038/s41573-021-00233-1. Epub 2021 Jun 30. Nat Rev Drug Discov. 2021. PMID: 34194012 Free PMC article. Review. - Intramuscular mechanisms of overtraining.
Cheng AJ, Jude B, Lanner JT. Cheng AJ, et al. Redox Biol. 2020 Aug;35:101480. doi: 10.1016/j.redox.2020.101480. Epub 2020 Feb 26. Redox Biol. 2020. PMID: 32179050 Free PMC article. Review. - Skeletal muscle redox signaling in rheumatoid arthritis.
Steinz MM, Santos-Alves E, Lanner JT. Steinz MM, et al. Clin Sci (Lond). 2020 Nov 13;134(21):2835-2850. doi: 10.1042/CS20190728. Clin Sci (Lond). 2020. PMID: 33146370 Free PMC article. Review.
References
- Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Pulmonary function in primary pulmonary hypertension. J Am Coll Cardiol. 2003;41:1028–35. Epub 2003/03/26. - PubMed
- Kabitz HJ, Schwoerer A, Bremer HC, Sonntag F, Walterspacher S, Walker D, et al. Impairment of respiratory muscle function in pulmonary hypertension. Clin Sci (Lond). 2008;114:165–71. Epub 2007/09/04. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical