Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration - PubMed (original) (raw)
. 2017 Jun 1;20(6):771-784.e6.
doi: 10.1016/j.stem.2017.02.009. Epub 2017 Mar 16.
Antonio Scialdone 2, Antonia Graja 1, Sabrina Gohlke 1, Anne-Marie Jank 1, Carla Bocian 1, Lena Woelk 1, Hua Fan 3, Darren W Logan 4, Annette Schürmann 5, Luis R Saraiva 6, Tim J Schulz 7
Affiliations
- PMID: 28330582
- PMCID: PMC5459794
- DOI: 10.1016/j.stem.2017.02.009
Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration
Thomas H Ambrosi et al. Cell Stem Cell. 2017.
Abstract
Aging and obesity induce ectopic adipocyte accumulation in bone marrow cavities. This process is thought to impair osteogenic and hematopoietic regeneration. Here we specify the cellular identities of the adipogenic and osteogenic lineages of the bone. While aging impairs the osteogenic lineage, high-fat diet feeding activates expansion of the adipogenic lineage, an effect that is significantly enhanced in aged animals. We further describe a mesenchymal sub-population with stem cell-like characteristics that gives rise to both lineages and, at the same time, acts as a principal component of the hematopoietic niche by promoting competitive repopulation following lethal irradiation. Conversely, bone-resident cells committed to the adipocytic lineage inhibit hematopoiesis and bone healing, potentially by producing excessive amounts of Dipeptidyl peptidase-4, a protease that is a target of diabetes therapies. These studies delineate the molecular identity of the bone-resident adipocytic lineage, and they establish its involvement in age-dependent dysfunction of bone and hematopoietic regeneration.
Keywords: DPP4; adipogenesis; adipogenic progenitors; aging; bone healing; bone marrow adipose tissue; hematopoiesis; mesenchymal stem cells; obesity; regeneration.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
Bone Marrow-Resident Mesenchymal Cells Display Functional Heterogeneity (A and B) Flow cytometric separation of CD31−CD45− cells by Sca1 selection, followed by (B) Oil Red O (adipogenesis), Alizarin Red S (osteogenesis), and Alcian Blue (chondrogenesis) staining of Sca1+ and Sca1− cells differentiated under the corresponding conditions. (C and D) FACS analyses (as in A) of CD31−CD45−Sca1+ cells separated by CD24 expression, and (D) subsequent differentiation of cells (as in B). (E) Representative image of clonal analysis with feeder cells by co-staining of clone 19-derived tdTomato+ cells with Perilipin, Osteocalcin, or Aggrecan to show lineage-specific differentiation potential. (F) In vivo luciferase imaging (top panels) and macroscopic identification (arrows; lower panels) of transplants 8 weeks after sternal subcutaneous (s.c.) injection of the indicated cell populations isolated from repAdiLuc mice. (G) Microscopy of corresponding Movat-Pentachrome stains (yellow, mineralized structure; blue, cartilage; purple, nuclei. Scale bars, 30 μm. See also Figures S1–S3 and Tables S1–S4.
Figure 2
Lineage Tracing of MAT Reveals a Mesenchymal, Non-endothelial, Non-hematopoietic Lineage (A) Representative merged IF images of bone-resident adipocytes from the indicated Cre-mTmG reporter mouse strains (green, EGFP; red, Perilipin; blue, DAPI). (B) Reporter analysis of bone lining cells and cortical bone-resident osteocytes (blue arrows; green, GFP; red, tdTomato; blue, DAPI). (C) FACS analysis of tdTomato+ and GFP+ cells in the CD45−CD31−Sca1+ population (percentages represent average values). (D) Quantification of GFP+ bone-resident adipocytes, bone lining cells, and osteocytes by image quantification and flow cytometric analysis of bone stroma cells populations (n = 3–4 mice/genotype). Mean ± SEM. Scale bars, 10 μm. See also Figure S3.
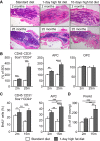
Figure 3
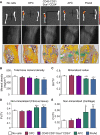
Aging and High-Fat Diet Stimulate Expansion of the Adipocytic Lineage (A) H&E stains of femora from male mice aged 2 (young) or 25 months (old) maintained on standard diet (SD) or high-fat diet (HFD) for 24 hr (1dHFD) or 10 days (10dHFD). (B) Relative quantifications of CD31−CD45−Sca1+CD24+, APC, and OPC populations in young and 25-month-old mice on SD (white bars) compared to 1dHFD (gray bars, applies to all panels) (n = 9). (C) BrdU incorporation into CD31−CD45−Sca1+CD24+ and APCs from young and 15-month-old mice on SD or 1dHFD (n = 7–9). (D) Quantification of GFP+ cells in 2-month- and 15-month-old male _Zfp423_-EGFP reporter mice on SD or 1dHFD (n = 6). Graphs show cumulative data from at least two independent experiments. Mean ± SEM; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. Scale bar, 100 μm. See also Figure S4.
Figure 4
Distinct Roles of Progenitor Populations during Hematopoietic Recovery (A) Schematic depiction of competitive hematopoietic repopulation assay. (B and C) H&E stains of injected tibiae (B), and tdTomato (red) and Perilipin (green) (C) IF co-localization on adipocytes in injected tibiae (green arrow, host derived; orange arrow, donor derived). Scale bars, 50 μm. (D) Fate analyses of transplanted cells. CD45−CD31−tdTomato+ cells (red squares, top row) were gated for expression of GFP (green squares, _Zfp423_-EGFP reporter; middle row). Zfp423+ cells were then assessed for expression of surface markers Sca1 and CD24 (bottom row). (E–G) Bone marrow cellularity (E), marrow chimerism (F), and donor-derived (G) LSK cells in injected and contralateral tibiae 5 weeks after irradiation. (H and I) Donor-derived LT-LSK (H) and donor-derived ST-LSK (I) hematopoietic stem cells in injected tibiae. Mean ± SEM (n = 7); p < 0.05: a, versus no cells; b, versus OPC; c, versus CD31−CD45−Sca1+CD24+; d, versus APC; and e, versus preAd. See also Figure S5.
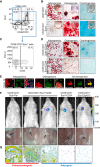
Figure 5
The Adipocytic Lineage Inhibits Bone Healing (A) Red fluorescence in tibiae (top panels), μCT images (middle panels), and representative Movat-Pentachrome stains (lower panels; yellow, mineralized bone; red, new bone; blue, cartilage; purple, nuclei) of fracture calluses 14 days after fracture and intratibial injection of the indicated cell populations. (B) Total bone mineral density (BMD) by μCT (n = 6). (C–E) Histomorphometry of mineralized bone volume (BV) and non-mineralized callus (C) as fibrous tissue (FV) (D), and cartilage tissue (CV) volumes (E), all normalized to total callus volume (TV) (n = 8). Mean ± SEM; p < 0.05: a, versus no cells; b, versus OPC; and c, versus CD31−CD45−Sca1+CD24+. Scale bar, 100 μm. See also Figure S6.
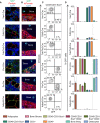
Figure 6
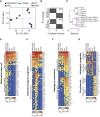
RNA-Seq Defines the Cellular Identities of Bone-Resident Sub-populations (A–C) The principal-component analysis (PCA; A), correlations scores (B) of the top ten genes driving PC1 and PC2 in (A), and hierarchical clustering analyses (C) of RNA-seq from all four cell populations. (D–G) Heatmaps of selected differentially expressed (DE) genes, divided by candidates reported in the literature (known, asterisks indicate no significant DE between individual groups) and novel markers, enriched in CD31−CD45−Sca1+CD24+ (D), OPC (E), APCs and preAds combined (F), and APC or preAd (G) cell populations. See also Figure S7 and Table S5.
Figure 7
DPP4 Inhibition Reverses the Negative Effects of Adipogenic Cells on Bone Regeneration (A) Gene expression intensities of Dpp4 from RNA-seq analysis. (B) _Dpp4_-mRNA levels in whole proximal and distal tibiae of young (2 months) and old (15 months) male mice (n = 5). (C) DPP4 secretion by whole tibia explants from young and old mice (n = 4). (D and E) The mRNA levels (D) of Runx2 and Osterix (Osx/Sp7) and Alizarin Red S staining and quantification (E) of CD45−CD31−Sca1+CD24+ and OPCs either treated with PBS (control, white bars) or sitagliptin (100 μM, black bars) during osteogenic differentiation (n = 3). Scale bars, 30 μm. (F) FACS analysis of OPC, CD45−CD31−Sca1+CD24+, and APC cell frequencies in tibiae from male mice either i.p. injected with PBS (white bars) or sitagliptin for 9 days (black bars; n = 9–10). Mean ± SEM; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗∗p < 0.0001. (G–I) Representative Movat-Pentachrome stains (G) of fracture calluses from control PBS-treated mice that received osteogenic (PBS/OPC) or adipogenic (PBS/APC) intratibial transplants and animals treated with sitagliptin for 1 week after fracture and receiving the same transplants of osteogenic (Sita/OPC) or adipogenic (Sita/APC) cells. Fracture callus total volumes (TVs) were analyzed for mineralized callus volume (H, BV/TV) and fibrous tissue volumes (I, FV/TV) (n = 5–7). (J–L) Representative Movat-Pentachrome stains and histomorphometric analyses (as in G–I) of fracture calluses 14 days after injury from mice that received osteogenic (OPC) or adipogenic (APC) intratibial transplants from either DPP4-KO or wild-type (WT) animals (n = 9). Mean ± SEM; p < 0.05: a, versus PBS or WT/OPC; b, versus PBS or WT/APC; c, versus sitagliptin or DPP4-KO/OPC; and d, versus sitagliptin or DPP4-KO/APC. Scale bars, 100 μm. See also Figure S7 and Table S6.
Comment in
- Adipocytes at the Core of Bone Function.
Grandl G, Wolfrum C. Grandl G, et al. Cell Stem Cell. 2017 Jun 1;20(6):739-740. doi: 10.1016/j.stem.2017.05.008. Cell Stem Cell. 2017. PMID: 28575686
Similar articles
- Inhibition of dipeptidyl peptidase 8/9 impairs preadipocyte differentiation.
Han R, Wang X, Bachovchin W, Zukowska Z, Osborn JW. Han R, et al. Sci Rep. 2015 Aug 5;5:12348. doi: 10.1038/srep12348. Sci Rep. 2015. PMID: 26242871 Free PMC article. - Bone marrow adipose cells - cellular interactions and changes with obesity.
Boroumand P, Klip A. Boroumand P, et al. J Cell Sci. 2020 Mar 6;133(5):jcs238394. doi: 10.1242/jcs.238394. J Cell Sci. 2020. PMID: 32144195 Review. - Identification of a mesenchymal progenitor cell hierarchy in adipose tissue.
Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, Percec I, Seale P. Merrick D, et al. Science. 2019 Apr 26;364(6438):eaav2501. doi: 10.1126/science.aav2501. Science. 2019. PMID: 31023895 Free PMC article. - Adipocytes at the Core of Bone Function.
Grandl G, Wolfrum C. Grandl G, et al. Cell Stem Cell. 2017 Jun 1;20(6):739-740. doi: 10.1016/j.stem.2017.05.008. Cell Stem Cell. 2017. PMID: 28575686 - Bone Marrow Adipose Tissue: Regulation of Osteoblastic Niche, Hematopoiesis and Hematological Malignancies.
Labella R, Vujačić M, Trivanović D. Labella R, et al. Stem Cell Rev Rep. 2023 Jul;19(5):1135-1151. doi: 10.1007/s12015-023-10531-3. Epub 2023 Mar 17. Stem Cell Rev Rep. 2023. PMID: 36930385 Review.
Cited by
- The Dynamic Interface Between the Bone Marrow Vascular Niche and Hematopoietic Stem Cells in Myeloid Malignancy.
Mosteo L, Storer J, Batta K, Searle EJ, Duarte D, Wiseman DH. Mosteo L, et al. Front Cell Dev Biol. 2021 Mar 11;9:635189. doi: 10.3389/fcell.2021.635189. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33777944 Free PMC article. Review. - Bidirectional interplay between metabolism and epigenetics in hematopoietic stem cells and leukemia.
Zhang YW, Schönberger K, Cabezas-Wallscheid N. Zhang YW, et al. EMBO J. 2023 Dec 11;42(24):e112348. doi: 10.15252/embj.2022112348. Epub 2023 Nov 27. EMBO J. 2023. PMID: 38010205 Free PMC article. Review. - RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss.
Hu Y, Li X, Zhi X, Cong W, Huang B, Chen H, Wang Y, Li Y, Wang L, Fang C, Guo J, Liu Y, Cui J, Cao L, Weng W, Zhou Q, Wang S, Chen X, Su J. Hu Y, et al. EMBO Rep. 2021 Jul 5;22(7):e52481. doi: 10.15252/embr.202152481. Epub 2021 Jun 13. EMBO Rep. 2021. PMID: 34121311 Free PMC article. - Changes in interstitial fluid flow, mass transport and the bone cell response in microgravity and normogravity.
Wei F, Flowerdew K, Kinzel M, Perotti LE, Asiatico J, Omer M, Hovell C, Reumers V, Coathup MJ. Wei F, et al. Bone Res. 2022 Nov 21;10(1):65. doi: 10.1038/s41413-022-00234-9. Bone Res. 2022. PMID: 36411278 Free PMC article. Review. - Impact of intestinal microenvironments in obesity and bariatric surgery on shaping macrophages.
Leyderman M, Wilmore JR, Shope T, Cooney RN, Urao N. Leyderman M, et al. Immunometabolism (Cobham). 2023 Nov 28;5(4):e00033. doi: 10.1097/IN9.0000000000000033. eCollection 2023 Oct. Immunometabolism (Cobham). 2023. PMID: 38037591 Free PMC article. Review.
References
- Carnevale V., Romagnoli E., D’Erasmo L., D’Erasmo E. Bone damage in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2014;24:1151–1157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous