Acoustic Enhancement of Sleep Slow Oscillations and Concomitant Memory Improvement in Older Adults - PubMed (original) (raw)
Acoustic Enhancement of Sleep Slow Oscillations and Concomitant Memory Improvement in Older Adults
Nelly A Papalambros et al. Front Hum Neurosci. 2017.
Abstract
Acoustic stimulation methods applied during sleep in young adults can increase slow wave activity (SWA) and improve sleep-dependent memory retention. It is unknown whether this approach enhances SWA and memory in older adults, who generally have reduced SWA compared to younger adults. Additionally, older adults are at risk for age-related cognitive impairment and therefore may benefit from non-invasive interventions. The aim of this study was to determine if acoustic stimulation can increase SWA and improve declarative memory in healthy older adults. Thirteen participants 60-84 years old completed one night of acoustic stimulation and one night of sham stimulation in random order. During sleep, a real-time algorithm using an adaptive phase-locked loop modeled the phase of endogenous slow waves in midline frontopolar electroencephalographic recordings. Pulses of pink noise were delivered when the upstate of the slow wave was predicted. Each interval of five pulses ("ON interval") was followed by a pause of approximately equal length ("OFF interval"). SWA during the entire sleep period was similar between stimulation and sham conditions, whereas SWA and spindle activity were increased during ON intervals compared to matched periods during the sham night. The increases in SWA and spindle activity were sustained across almost the entire five-pulse ON interval compared to matched sham periods. Verbal paired-associate memory was tested before and after sleep. Overnight improvement in word recall was significantly greater with acoustic stimulation compared to sham and was correlated with changes in SWA between ON and OFF intervals. Using the phase-locked-loop method to precisely target acoustic stimulation to the upstate of sleep slow oscillations, we were able to enhance SWA and improve sleep-dependent memory storage in older adults, which strengthens the theoretical link between sleep and age-related memory integrity.
Keywords: acoustic stimulation; aging; memory; sleep; slow waves.
Figures
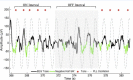
FIGURE 1
Excerpt of data for phase-locked loop (PLL) pulse delivery for ON and OFF intervals. The PLL continuously models phase (gray-dotted line) to target the upstate of the slow wave. The acoustic stimulation is delivered in blocks of five pulses ∼1.2 s apart (red dots, ON Interval) followed by an off period of ∼6 s (OFF Interval). PLL, Phase-locked loop; SW, slow wave.
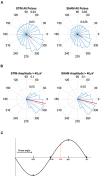
FIGURE 2
Circular histograms for STIM and SHAM conditions averaged across all participants. (A) Phase spread of all pulses, including pulses that inadvertently hit other phases. Red lines indicate mean phase vector and spread is depicted in 20 bins of 18 degrees. During the SHAM night, time of pulses was marked, but no sound was played. (B) Phase spread of all pulses for slow waves with amplitudes greater than 40 μV for both the STIM and SHAM. (C) Graphical illustration of phase angle, dotted red line indicates location of target phase on the slow wave.
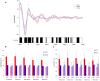
FIGURE 3
Overlay of auditory ERPs for STIM and SHAM nights. (A) Grand average ERPs for the STIM (red) and SHAM (blue) nights aligned to the first pulse of each ON interval (time = 0). Black bars indicate p < 0.05 between STIM and SHAM conditions. (B) Average peak-to-peak amplitude of 1.5 s ERPs windows, time locked to pulse one through five, for STIM and SHAM ON and OFF intervals. (C) Average slow-wave peak-to-peak amplitudes ≥75 μV for the 1.5 s ERP windows time locked to pulse one through five.
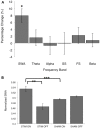
FIGURE 4
Normalized spectral power for the STIM and SHAM periods. (A) Percentage change from the SHAM ON to the STIM ON condition for SWA (>0.5–4 Hz), theta (>4–8 Hz), alpha (>8–12 Hz), slow spindle (SS, >11–13 Hz), fast spindle (FS, >13–15 Hz), and beta (>16–20 Hz) frequency bands. The change in SWA was significantly increased following STIM compared to SHAM (∗p = 0.006). (B) SWA for the STIM and SHAM, ON and OFF intervals. ∗∗p = 0.006, ∗∗∗p = 0.002 for paired _t_-tests.
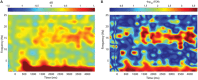
FIGURE 5
Change in spectral power between STIM and SHAM conditions. (A) Frequency by time plot for the STIM – SHAM ON intervals at Fpz, time locked to the onset of each five-pulse interval (time = 0). There was a robust increase in SWA, with smaller changes in theta activity beginning 500 ms after sound delivery. A modest increase in spindle activity was seen beginning at 1000 ms, with the greatest change at 3000 ms after sound delivery. (B) Significance of spectral power differences between the STIM ON and SHAM ON intervals. Color corresponds to the FDR-corrected _p_-values on a negative log scale, with dark blue corresponding to least significant (FDR = 1) and red corresponding to most significant (FDR = 0.0002).
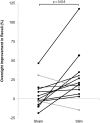
FIGURE 6
Overnight percent improvement in recall for each subject for the STIM and SHAM conditions. A positive value indicates memory improvement. Black indicates participants with a larger overnight improvement in recall in the STIM compared to the SHAM condition. Gray represents two participants who performed worse in the STIM compared to the SHAM condition. The increase in word pair recall following STIM was significantly higher than SHAM (p = 0.016).
FIGURE 7
Correlation between overnight percent improvement in recall between nights and the percent change in SWA for the ON and OFF intervals between nights (% change STIM – % change SHAM, r = 0.64, p = 0.018).
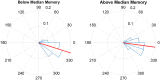
FIGURE 8
Pulse delivery was significantly closer to the upstate (360/0°) in participants with above-median improvement in memory between the STIM and SHAM night. Above-median (n = 7) memory performance was defined as ≥17% improvement in word recall and below-median (n = 6) improvement was defined as < 17% improvement. Circular y-axis indicates normalized counts. Red lines indicate mean vector, while blue triangles indicate bin spread (p < 0.0001).
Similar articles
- Acoustic enhancement of sleep slow oscillations in mild cognitive impairment.
Papalambros NA, Weintraub S, Chen T, Grimaldi D, Santostasi G, Paller KA, Zee PC, Malkani RG. Papalambros NA, et al. Ann Clin Transl Neurol. 2019 Jul;6(7):1191-1201. doi: 10.1002/acn3.796. Epub 2019 Jul 1. Ann Clin Transl Neurol. 2019. PMID: 31353857 Free PMC article. Clinical Trial. - Acoustic slow wave sleep enhancement via a novel, automated device improves executive function in middle-aged men.
Diep C, Ftouni S, Manousakis JE, Nicholas CL, Drummond SPA, Anderson C. Diep C, et al. Sleep. 2020 Jan 13;43(1):zsz197. doi: 10.1093/sleep/zsz197. Sleep. 2020. PMID: 31691831 Clinical Trial. - Boosting Slow Oscillatory Activity Using tDCS during Early Nocturnal Slow Wave Sleep Does Not Improve Memory Consolidation in Healthy Older Adults.
Paßmann S, Külzow N, Ladenbauer J, Antonenko D, Grittner U, Tamm S, Flöel A. Paßmann S, et al. Brain Stimul. 2016 Sep-Oct;9(5):730-739. doi: 10.1016/j.brs.2016.04.016. Epub 2016 Apr 28. Brain Stimul. 2016. PMID: 27247261 Clinical Trial. - Can Slow-Wave Sleep Enhancement Improve Memory? A Review of Current Approaches and Cognitive Outcomes.
Zhang Y, Gruber R. Zhang Y, et al. Yale J Biol Med. 2019 Mar 25;92(1):63-80. eCollection 2019 Mar. Yale J Biol Med. 2019. PMID: 30923474 Free PMC article. Review. - Neurostimulation techniques to enhance sleep and improve cognition in aging.
Grimaldi D, Papalambros NA, Zee PC, Malkani RG. Grimaldi D, et al. Neurobiol Dis. 2020 Jul;141:104865. doi: 10.1016/j.nbd.2020.104865. Epub 2020 Apr 4. Neurobiol Dis. 2020. PMID: 32251840 Review.
Cited by
- Are age and sex effects on sleep slow waves only a matter of electroencephalogram amplitude?
Rosinvil T, Bouvier J, Dubé J, Lafrenière A, Bouchard M, Cyr-Cronier J, Gosselin N, Carrier J, Lina JM. Rosinvil T, et al. Sleep. 2021 Mar 12;44(3):zsaa186. doi: 10.1093/sleep/zsaa186. Sleep. 2021. PMID: 32929490 Free PMC article. - Acoustically evoked K-complexes together with sleep spindles boost verbal declarative memory consolidation in healthy adults.
Leach S, Krugliakova E, Sousouri G, Snipes S, Skorucak J, Schühle S, Müller M, Ferster ML, Da Poian G, Karlen W, Huber R. Leach S, et al. Sci Rep. 2024 Aug 19;14(1):19184. doi: 10.1038/s41598-024-67701-7. Sci Rep. 2024. PMID: 39160150 Free PMC article. - Auditory or Audiovisual Stimulation Ameliorates Cognitive Impairment and Neuropathology in ApoE4 Knock-In Mice.
Jung H, Lee Y, Lee SH, Sohn JH. Jung H, et al. Int J Mol Sci. 2023 Jan 4;24(2):938. doi: 10.3390/ijms24020938. Int J Mol Sci. 2023. PMID: 36674449 Free PMC article. - Sleep from acute to chronic traumatic brain injury and cognitive outcomes.
Sanchez E, Blais H, Duclos C, Arbour C, Van Der Maren S, El-Khatib H, Baril AA, Bernard F, Carrier J, Gosselin N. Sanchez E, et al. Sleep. 2022 Aug 11;45(8):zsac123. doi: 10.1093/sleep/zsac123. Sleep. 2022. PMID: 35640250 Free PMC article. - Auditory deep sleep stimulation in older adults at home: a randomized crossover trial.
Lustenberger C, Ferster ML, Huwiler S, Brogli L, Werth E, Huber R, Karlen W. Lustenberger C, et al. Commun Med (Lond). 2022 Apr 4;2:30. doi: 10.1038/s43856-022-00096-6. eCollection 2022. Commun Med (Lond). 2022. PMID: 35603302 Free PMC article.
References
- Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 1–48. 10.18637/jss.v067.i01 - DOI
- Berens P. (2009). CircStat: a Matlab toolbox for circular statistics. J. Stat. Softw. 31 1–21. 10.18637/jss.v031.i10 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous