HLA-DP84Gly constitutively presents endogenous peptides generated by the class I antigen processing pathway - PubMed (original) (raw)
doi: 10.1038/ncomms15244.
Mark Anczurowski 1 2, Munehide Nakatsugawa 1, Makito Tanaka 3, Yuki Kagoya 1, Ankit Sinha 4 5, Kenji Chamoto 1, Toshiki Ochi 1, Tingxi Guo 1 2, Kayoko Saso 1, Marcus O Butler 1 2 6, Mark D Minden 7 8, Thomas Kislinger 4 5, Naoto Hirano 1 2
Affiliations
- PMID: 28489076
- PMCID: PMC5436232
- DOI: 10.1038/ncomms15244
HLA-DP84Gly constitutively presents endogenous peptides generated by the class I antigen processing pathway
Yuki Yamashita et al. Nat Commun. 2017.
Abstract
Classical antigen processing leads to the presentation of antigenic peptides derived from endogenous and exogenous sources for MHC class I and class II molecules, respectively. Here we show that, unlike other class II molecules, prevalent HLA-DP molecules with β-chains encoding Gly84 (DP84Gly) constitutively present endogenous peptides. DP84Gly does not bind invariant chain (Ii) via the class II-associated invariant chain peptide (CLIP) region, nor does it present CLIP. However, Ii does facilitate the transport of DP84Gly from the endoplasmic reticulum (ER) to the endosomal/lysosomal pathway by transiently binding DP84Gly via a non-CLIP region(s) in a pH-sensitive manner. Accordingly, like class I, DP84Gly constitutively presents endogenous peptides processed by the proteasome and transported to the ER by the transporter associated with antigen processing (TAP). Therefore, DP84Gly, found only in common chimpanzees and humans, uniquely uses both class I and II antigen-processing pathways to present peptides derived from intracellular and extracellular sources.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
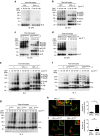
Figure 1. HLA-DP molecules whose β-chains encode Gly84 are unable to present CLIP.
Surface class II, Ii and CLIP expression on T2 transfectants were analysed by flow cytometry following staining with specific monoclonal antibodies (mAbs). Note that T2 cells constitutively express Ii. (a,b) T2 cells were stably transduced with DPA1*01:03 (DPA1; a) or DPA1*02:01 (DPA2; b) in conjunction with DPB1*02:01(DPB2), DPB1*04:01 (DPB4), DPB1*05:01 (DPB5) or DPB1*08:01 (DPB8). (c) T2 cells were stably transfected with DPA1 along with mutated DPB284DEAV87 (DP284DEAV87), DPB484DEAV87 (DP484DEAV87), DPB584GGPM87 (DP584GGPM87) or DPB884GGPM87 (DP884GGPM87), whose amino-acid residues at positions 84–87 of the DPβ chain were substituted as indicated. (d) T2 cells were stably transfected with DPA1 in conjunction with mutant DPB284Asp (DP284Asp), DPB484Asp (DP484Asp), DPB584Gly (DP584Gly) or DPB884Gly (DP884Gly), where point mutations of the amino-acid residue at position 84 of each DPβ chain were substituted as indicated.
Figure 2. CLIP is produced by DR and DP5 but not DP4.
(a–c) Total cell lysates were immunoprecipitated and immunoblotted with indicated mAbs. Total cell lysates were prepared from T2 transfectants expressing the indicated class II as a single class II allele (a,b) and EBV-LCL homozygous for DP84DEAV87 (DP5/17) or DP84GGPM87 (DP4/4) (c).
Figure 3. DP84DEAV87 but not DP84GGPM87 molecules form a nonamer complex with Ii via the CLIP region.
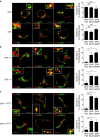
(a–g) HEK293 cells were transiently transfected with the indicated combinations of genes. Cells were treated with DSP, a chemical crosslinker, at the indicated concentrations for 2 h. Non-reduced samples were immunoblotted with anti-DPβ (a–d) or Ii (e–g) mAb. (h,i) HEK293 cells were transfected with the indicated combinations of genes. Fixed cells were permeabilized and stained for Ii (h) or IiR-CLIP (i; green) and DP (red), and then analysed by confocal microscopy. Inset boxes indicate the areas shown at higher magnification. Note that HEK293 cells are deficient in class II and Ii expression. Scale bar in all images, 10 μm. Quantification of co-localized spots represents means±s.d. of three counted cells in each condition. ns, not significant; *P<0.05 by unpaired, two-tailed Welch's _t_-test.
Figure 4. Subcellular localization of DP4 and DP5 in the presence or absence of Ii.
(a–c) HEK293 cells were transfected with the indicated combinations of genes. Fixed cells were permeabilized and stained for DP (red) in conjunction with an ER marker, PDI (green, left panels), an early endosomal marker, EEA1 (green, middle panels) or a late endosomal/lysosomal marker, LAMP-1 (green, right panels) and analysed by confocal microscopy. Inset boxes indicate the areas depicted at higher magnification. Scale bar in all images, 10 μm. Quantification of co-localized spots represents means±s.d. of three counted cells in each condition. ns, not significant; *P<0.05, **P<0.01, ***P<0.001 by unpaired, two-tailed Welch's _t_-test.
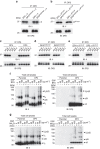
Figure 5. DP84GGPM87 binds to Ii via a non-CLIP region(s) in neutral pH conditions.
(a,b) HEK293 cells were transfected with the indicated combinations of genes. Total cell lysates were immunoprecipitated with anti-DPβ mAb and immunoblotted with anti-Ii or DPβ mAb. (c–e) Lysates of T2/DP transfectants (c,d) or HEK293 cells transfected with the indicated combinations of DP and IiR-CLIP genes (e) were immunoprecipitated with anti-DPβ mAb. Note that T2 cells naturally express Ii. The immunoprecipitates were washed with buffer of graded pH as indicated and immunoblotted with anti-Ii or DPβ mAb. (f,g) T2 and T2/DP4 were cultured in the presence or absence of 10 μg ml−1 BFA (f) or 40 mM NH4Cl (g). Cells were further treated with DSP at the indicated concentrations for 2 h. Non-reduced samples were immunoblotted with anti-Ii (left) or DPβ (right) mAb.
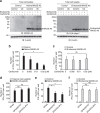
Figure 6. DP84GGPM87-expressing cells constitutively present intracellular peptides generated by the proteasome and TAP-dependent pathway.
(a) K562 aAPCs were transiently transfected with the indicated combinations of genes and cultured in the presence or absence of 0.02 μM bortezomib or 0.02 μM carfilzomib for 48 h. Total cell lysates were immunoblotted with anti-MAGE-A3, anti-HLA-class I or anti-β-actin mAb. (b,c) K562/DP4/Ii cells were transiently transfected with a retrovirus vector encoding IRES-EGFP (control), or a native (b) or endosome-targeted (c) form of MAGE-A3 linked with IRES-EGFP. Cells were then cultured with carfilzomib at the indicated concentrations for 48 h. Transient transfection efficiencies were normalized to EGFP expression measured by flow cytometry. DP4/MAGE-A3243–258 CD4+ T cells were stimulated with the K562/DP4/Ii transfectants and IFN-γ secretion was measured by ELISPOT analysis. Data shown represent means±s.d.'s of triplicates. (d) DP4/MAGE-A3243–258 CD4+ T cells were stimulated with the indicated K562-based aAPCs pulsed with tetanus toxin947–967 (control) or MAGE-A3243–258 peptide, and IFN-γ secretion was evaluated by ELISPOT assays. Data shown represent means±s.d.'s of triplicates. (e,f) The indicated K562-based aAPCs were transiently transfected with a retrovirus vector encoding IRES-EGFP (control) or a native (e) or endosome-targeted (f) form of MAGE-A3 linked with IRES-EGFP. Transient transfection efficiencies were normalized to EGFP expression measured by flow cytometry. DP4/MAGE-A3243–258 CD4+ T cells were stimulated with the indicated aAPCs and IFN-γ secretion was measured by ELISPOT analysis. Data shown represent means±s.d.'s of triplicates. Results are representative of three independent experiments. ns, not significant; *P<0.05 by unpaired, two-tailed Welch's _t_-test.
Figure 7. DP84GGPM87 but not DP84DEAV87 constitutively presents peptides derived from intracellular proteins regardless of Ii expression.
(a,b) The indicated K562-based aAPCs were transiently transfected with a retrovirus vector encoding IRES-EGFP (control) or a native (a) or endosome-targeted (b) form of MAGE-A3 linked with IRES-EGFP. Transient transfection efficiencies were normalized to EGFP expression measured by flow cytometry. DP4/MAGE-A3243–258 CD4+ T cells were stimulated with the indicated APCs and IL-2 secretion was measured by ELISPOT analysis. Data shown represent means±s.d.'s of triplicates. (c) NSG mice were subcutaneously inoculated with 2 × 106 K562 cells stably expressing DP4/Ii/MAGE-A3 or DP484DEAV87/Ii/MAGE-A3. Two days later, the mice were treated with 4 × 107 CD3+ T cells untransduced or transduced with DP4/MAGE-A3243–258 TCR. The mean tumour size for each group is represented as the average±s.d. of three mice. There was no significant difference in the tumorigenicity of the two cell lines (data not shown). Results are representative of three independent experiments. ns, not significant; *P<0.05, **P<0.01 by unpaired, two-tailed Welch's _t_-test.
Similar articles
- Mechanisms underlying the lack of endogenous processing and CLIP-mediated binding of the invariant chain by HLA-DP84Gly.
Anczurowski M, Yamashita Y, Nakatsugawa M, Ochi T, Kagoya Y, Guo T, Wang CH, Rahman MA, Saso K, Butler MO, Hirano N. Anczurowski M, et al. Sci Rep. 2018 Mar 19;8(1):4804. doi: 10.1038/s41598-018-22931-4. Sci Rep. 2018. PMID: 29555965 Free PMC article. - Invariant chain as a vehicle to load antigenic peptides on human MHC class I for cytotoxic T-cell activation.
Wälchli S, Kumari S, Fallang LE, Sand KM, Yang W, Landsverk OJ, Bakke O, Olweus J, Gregers TF. Wälchli S, et al. Eur J Immunol. 2014 Mar;44(3):774-84. doi: 10.1002/eji.201343671. Epub 2013 Dec 27. Eur J Immunol. 2014. PMID: 24293164 - Chaperones of the class I peptide-loading complex facilitate the constitutive presentation of endogenous antigens on HLA-DP84GGPM87.
Anczurowski M, Sugata K, Matsunaga Y, Yamashita Y, Wang CH, Guo T, Murata K, Saijo H, Kagoya Y, Saso K, Butler MO, Hirano N. Anczurowski M, et al. J Autoimmun. 2019 Aug;102:114-125. doi: 10.1016/j.jaut.2019.04.023. Epub 2019 May 8. J Autoimmun. 2019. PMID: 31078377 - Involvement of autophagy in MHC class I antigen presentation.
Øynebråten I. Øynebråten I. Scand J Immunol. 2020 Nov;92(5):e12978. doi: 10.1111/sji.12978. Epub 2020 Oct 19. Scand J Immunol. 2020. PMID: 32969499 Free PMC article. Review. - The role of H2-O and HLA-DO in major histocompatibility complex class II-restricted antigen processing and presentation.
Alfonso C, Liljedahl M, Winqvist O, Surh CD, Peterson PA, Fung-Leung WP, Karlsson L. Alfonso C, et al. Immunol Rev. 1999 Dec;172:255-66. doi: 10.1111/j.1600-065x.1999.tb01370.x. Immunol Rev. 1999. PMID: 10631951 Review.
Cited by
- A core group of structurally similar HLA-DPB1 alleles drives permissiveness after hematopoietic cell transplantation.
Arrieta-Bolaños E, Crivello P, He M, Wang T, Gadalla SM, Paczesny S, Marsh SGE, Lee SJ, Spellman SR, Bolon YT, Fleischhauer K. Arrieta-Bolaños E, et al. Blood. 2022 Aug 11;140(6):659-663. doi: 10.1182/blood.2022015708. Blood. 2022. PMID: 35609150 Free PMC article. No abstract available. - The transmembrane domain and luminal C-terminal region independently support invariant chain trimerization and assembly with MHCII into nonamers.
Cloutier M, Fortin JS, Thibodeau J. Cloutier M, et al. BMC Immunol. 2021 Aug 12;22(1):56. doi: 10.1186/s12865-021-00444-6. BMC Immunol. 2021. PMID: 34384367 Free PMC article. - HTLV-1 infection promotes excessive T cell activation and transformation into adult T cell leukemia/lymphoma.
Tan BJ, Sugata K, Reda O, Matsuo M, Uchiyama K, Miyazato P, Hahaut V, Yamagishi M, Uchimaru K, Suzuki Y, Ueno T, Suzushima H, Katsuya H, Tokunaga M, Uchiyama Y, Nakamura H, Sueoka E, Utsunomiya A, Ono M, Satou Y. Tan BJ, et al. J Clin Invest. 2021 Dec 15;131(24):e150472. doi: 10.1172/JCI150472. J Clin Invest. 2021. PMID: 34907908 Free PMC article. - Variation and expression of HLA-DPB1 gene in HBV infection.
Ou G, Liu X, Xu H, Ji X, Liu X, Wang J. Ou G, et al. Immunogenetics. 2021 Jun;73(3):253-261. doi: 10.1007/s00251-021-01213-w. Epub 2021 Mar 12. Immunogenetics. 2021. PMID: 33710355 - NKp44/HLA-DP-dependent regulation of CD8 effector T cells by NK cells.
Padoan B, Casar C, Krause J, Schultheiss C, Baumdick ME, Niehrs A, Zecher BF, Pujantell M, Yuki Y, Martin M, Remmerswaal EBM, Dekker T, van der Bom-Baylon ND, Noble JA, Carrington M, Bemelman FJ, van Lier RAW, Binder M, Gagliani N, Bunders MJ, Altfeld M. Padoan B, et al. Cell Rep. 2024 Apr 23;43(4):114089. doi: 10.1016/j.celrep.2024.114089. Epub 2024 Apr 13. Cell Rep. 2024. PMID: 38615318 Free PMC article.
References
- Neefjes J., Jongsma M. L., Paul P. & Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 (2011). - PubMed
- Roche P. A., Marks M. S. & Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature 354, 392–394 (1991). - PubMed
- Lamb C. A. & Cresswell P. Assembly and transport properties of invariant chain trimers and HLA-DR-invariant chain complexes. J. Immunol. 148, 3478–3482 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous