Autophagy is essential for hearing in mice - PubMed (original) (raw)
Autophagy is essential for hearing in mice
Chisato Fujimoto et al. Cell Death Dis. 2017.
Abstract
Hearing loss is the most frequent sensory disorder in humans. Auditory hair cells (HCs) are postmitotic at late-embryonic differentiation and postnatal stages, and their damage is the major cause of hearing loss. There is no measurable HC regeneration in the mammalian cochlea, and the maintenance of cell function is crucial for preservation of hearing. Here we generated mice deficient in autophagy-related 5 (Atg5), a gene essential for autophagy, in the HCs to investigate the effect of basal autophagy on hearing acuity. Deletion of Atg5 resulted in HC degeneration and profound congenital hearing loss. In autophagy-deficient HCs, polyubiquitinated proteins and p62/SQSTM1, an autophagy substrate, accumulated as inclusion bodies during the first postnatal week, and these aggregates increased in number. These findings revealed that basal autophagy has an important role in maintenance of HC morphology and hearing acuity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
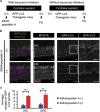
Basal autophagy flux was detected in auditory HCs. (a) Experimental design after the administration of lysosomal protease inhibitors, E64d and pepstatin A, to cochlear explant cultures established from P5 GFP-LC3 transgenic mice. Fixation was performed 4 h after the administration of lysosomal protease inhibitors. (b) Basal autophagy flux in the auditory HCs. GFP-LC3 puncta in the HC cytoplasm in a cochlear explant culture established from P5 GFP-LC3 transgenic mice were monitored. The accumulation of GFP-LC3 puncta was increased in cultures treated with E64d and pepstatin A for 4 h in comparison with cultures without these inhibitors. MYO7A was used as a marker of the HC cytoplasm. Magnified images of GFP-LC3 staining are shown in the rightmost panels. IHC, inner HC; OHC, outer HC. Scale bars: 10 _μ_m. (c) Quantitative analysis of the accumulation of GFP-LC3 puncta. The average number of GFP-LC3 puncta was significantly increased in cultures treated with E64d and pepstatin A (_n_=5) in comparison with cultures without these inhibitors (_n_=5). Error bars: S.E. **P<0.01, ***P<0.001
Figure 2
Autophagy was inhibited in the HCs from Atg5 flox/flox ;Pou4f3-Cre mice. In the HCs from P5 Atg5 flox/+ ;Pou4f3-Cre;GFP-LC3 mice, a number of GFP-LC3 puncta (white arrow heads) were observed in the cochlear HCs. Conversely, P5 Atg5 flox/flox ;Pou4f3-Cre;GFP-LC3 mice showed almost no GFP-LC3 puncta in the HCs. MYO7A was used as a marker of the HC cytoplasm. Magnified images of GFP-LC3 staining are shown in the lowermost panels. The GFP-positive region was also observed at the auditory nerve fibers in both Atg5 flox/flox ;Pou4f3-Cre;GFP-LC3 and Atg5 flox/+ ;Pou4f3-Cre;GFP-LC3 mice. The possibility that this region indicated autophagy-related structures or aggregates could not be denied. IHC, inner HC; OHC, outer HC. Scale bars: 10 _μ_m
Figure 3
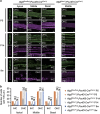
Aggregates containing ubiquitin and p62 were observed in the HCs from Atg5 flox/flox ;Pou4f3-Cre mice. (a) Aggregates in the HCs from Atg5 flox/flox ;Pou4f3-Cre mice. In the HCs from P5 Atg5 flox/+ ;Pou4f3-Cre mice, punctate signals positive for ubiquitin and/or p62 were not observed. In the HCs from P5 Atg5 flox/flox ;Pou4f3-Cre mice, aggregates containing ubiquitin and p62 were observed. At P14, these aggregates in Atg5 flox/flox ;Pou4f3-Cre mice became more massive. These additional aggregates were not generated in the HCs from Atg5 flox/+ ;Pou4f3-Cre mice. MYO7A was used as a marker of the HC cytoplasm. IHC, inner HC; OHC, outer HC. Scale bars: 10 _μ_m. (b–d) Quantitative analyses of the number of aggregates containing ubiquitin and p62 for the total HCs (b), the IHCs (c), and the OHCs (d). The number of aggregates in P5 Atg5 flox/flox ;Pou4f3-Cre mice (_n_=6) was significantly greater than that in P5 Atg5 flox/+ ;Pou4f3-Cre mice (_n_=6) for the total HCs (b), the IHCs (c), and the OHCs (d). The number of aggregates in P14 Atg5 flox/flox ;Pou4f3-Cre mice (_n_=6) was significantly greater than those in P14 Atg5 flox/+ ;Pou4f3-Cre mice (_n_=6) and P5 Atg5 flox/flox ;Pou4f3-Cre mice for the total HCs (b), the IHCs (c), and the OHCs (d). Error bars: S.E. NS, not significant, **P<0.01, ***P<0.001
Figure 4
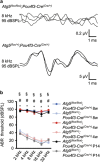
Atg5 flox/flox ;Pou4f3-Cre mice developed congenital severe hearing loss. (a) ABR waveform recordings over 10 ms to 8-kHz tone-burst stimuli from representative Atg5 flox/flox ;Pou4f3-Cre mice and Atg5 flox/+ ;Pou4f3-Cre mice at P14. Atg5 flox/flox ;Pou4f3-Cre mice showed reproducible waveforms and Atg5 flox/flox ;Pou4f3-Cre mice showed no waveforms at 95 dB sound pressure level (dBSPL). (b) A comparison of the ABR threshold between Atg5 flox/flox ;Pou4f3-Cre and Atg5 flox/+ ;Pou4f3-Cre mice was performed by Student’s _t_-test at P14, 4 weeks of age, and 8 weeks of age. When no response was obtained, we used 100 dBSPL as the threshold volume for calculations. Atg5 flox/flox ;Pou4f3-Cre mice showed profound congenital hearing loss already at P14 and thereafter and had significantly elevated hearing thresholds compared with Atg5 flox/+ ;Pou4f3-Cre mice. The following genotypes were tested at P14: Atg5 flox/flox ;Pou4f3-Cre (_n_=6) and Atg5 flox/+ ;Pou4f3-Cre (_n_=10); at 1 month: Atg5 flox/flox ;Pou4f3-Cre (_n_=6) and Atg5 flox/+ ;Pou4f3-Cre (_n_=6); and at 2 months: Atg5 flox/flox ;Pou4f3-Cre (_n_=6) and Atg5 flox/+ ;Pou4f3-Cre (n_=8). Error bars: S.E. *P<0.001 for the study at P14; #P<0.001 for the study at 4 weeks of age; §_P<0.001 for the study at 8 weeks of age
Figure 5
Damage to _Atg5_-deficient HCs was progressively developed. (a) Confocal imaging of the organ of Corti in Atg5 flox/flox ;Pou4f3-Cre and Atg5 flox/+ ;Pou4f3-Cre mice. The HCs in Atg5 flox/flox ;Pou4f3-Cre mice showed normal morphogenesis at P5. At P14, stereocilia were damaged in many HCs and some of the outer HC (OHC) bodies were destroyed. At 8 weeks of age, almost all of the OHC bodies and many inner HC (IHC) bodies were destroyed, and most of the stereocilia in the IHCs were damaged. MYO7A and phalloidin were used as markers of the HC cytoplasm and stereocilia, respectively. O1, the first row outer HC; O2, the second row outer HC; O3, the third row outer HC. Scale bars: 10 _μ_m. (b) A comparison of the number of cells per 100 _μ_m from Atg5 flox/flox ;Pou4f3-Cre mice (_n_=5) and Atg5 flox/+ ;Pou4f3-Cre mice (_n_=5) was performed by Student’s _t_-test at P5, P14, and 8 weeks of age. At P5, there were no significant differences between these genotypes. At P14 and 8 weeks of age, Atg5 flox/flox ;Pou4f3-Cre mice had significantly fewer HCs than Atg5 flox/+ ;Pou4f3-Cre mice. Error bars: S.E. **P<0.01, ***P<0.001
Figure 6
Stereocilia of _Atg5_-deficient HCs were progressively damaged. Scanning electron microscopy of the HCs in Atg5 flox/flox ;Pou4f3-Cre and Atg5 flox/+ ;Pou4f3-Cre mice. The HCs in Atg5 flox/flox ;Pou4f3-Cre mice showed normal morphogenesis at P5. At P14, stereocilia were damaged or irregularly shaped in many HCs. At 8 weeks of age in Atg5 flox/flox ;Pou4f3-Cre mice, almost all of the stereocilia in the outer HCs (OHCs) were destroyed and most of the stereocilia in the inner HCs were damaged or irregularly shaped. Scale bars: 10 _μ_m for total HCs, 1 _μ_m for OHC
Figure 7
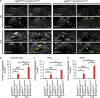
FM1-43 uptake into the HCs was not affected in P5 Atg5 flox/flox ;Pou4f3-Cre mice. (a) Experimental design of FM1-43 uptake assay in cochlear explant cultures established from P5 mice. FM1-43 uptake assay was performed after 2 h of incubation. (b) FM1-43 uptake in P5 cochlear explant cultures derived from Atg5 flox/flox ;Pou4f3-Cre and Atg5 flox/+ ;Pou4f3-Cre mice. Both Atg5 flox/flox ;Pou4f3-Cre and Atg5 flox/+ ;Pou4f3-Cre mice HCs displayed robust uptake of FM1-43, whereas both Atg5 flox/flox ;Pou4f3-Cre and Atg5 flox/+ ;Pou4f3-Cre mice HCs pretreated with 5 mM BAPTA did not take up FM1-43. (c) A comparison of the number of FM1-43-postitive HCs per 100 _μ_m between Atg5 flox/flox ;Pou4f3-Cre mice (_n_=5) and Atg5 flox/+ ;Pou4f3-Cre mice (_n_=5) was performed by Student’s _t_-test. There were no significant differences in the number of FM1-43-postitive HCs between Atg5 flox/flox ;Pou4f3-Cre and Atg5 flox/+ ;Pou4f3-Cre mice. IHC, inner HC; OHC, outer HC. Scale bars: 20 _μ_m. Error bars: S.E. NS, not significant
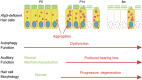
Figure 8
Schematic view of the phenotype in the HCs of Atg5 flox/flox ;Pou4f3-Cre mice based on morphological and functional analyses. In P5 HCs, no morphological changes were observed and mechanotransduction was normal, although aggregates containing ubiquitin and p62 were observed in the cytoplasm. At P14, aggregates became more massive. Stereocilia were damaged in many HCs and some of HC bodies were destroyed. P14 mice showed profound hearing loss. At 8 weeks of age, loss of HCs was progressively developed
Similar articles
- Auditory hair cell-specific deletion of p27Kip1 in postnatal mice promotes cell-autonomous generation of new hair cells and normal hearing.
Walters BJ, Liu Z, Crabtree M, Coak E, Cox BC, Zuo J. Walters BJ, et al. J Neurosci. 2014 Nov 19;34(47):15751-63. doi: 10.1523/JNEUROSCI.3200-14.2014. J Neurosci. 2014. PMID: 25411503 Free PMC article. - CNOT2 promotes degradation of p62/SQSTM1 as a negative regulator in ATG5 dependent autophagy.
Jeong K, Kwon HY, Jeong MS, Sohn EJ, Kim SH. Jeong K, et al. Oncotarget. 2017 Jul 11;8(28):46034-46046. doi: 10.18632/oncotarget.17682. Oncotarget. 2017. PMID: 28537904 Free PMC article. - Essential role of autophagy in protecting neonatal haematopoietic stem cells from oxidative stress in a p62-independent manner.
Nomura N, Ito C, Ooshio T, Tadokoro Y, Kohno S, Ueno M, Kobayashi M, Kasahara A, Takase Y, Kurayoshi K, Si S, Takahashi C, Komatsu M, Yanagawa T, Hirao A. Nomura N, et al. Sci Rep. 2021 Jan 18;11(1):1666. doi: 10.1038/s41598-021-81076-z. Sci Rep. 2021. PMID: 33462315 Free PMC article. - Ca2+ signaling, apoptosis and autophagy in the developing cochlea: Milestones to hearing acquisition.
Mammano F, Bortolozzi M. Mammano F, et al. Cell Calcium. 2018 Mar;70:117-126. doi: 10.1016/j.ceca.2017.05.006. Epub 2017 May 11. Cell Calcium. 2018. PMID: 28578918 Review. - Regulation of selective autophagy: the p62/SQSTM1 paradigm.
Lamark T, Svenning S, Johansen T. Lamark T, et al. Essays Biochem. 2017 Dec 12;61(6):609-624. doi: 10.1042/EBC20170035. Print 2017 Dec 12. Essays Biochem. 2017. PMID: 29233872 Review.
Cited by
- The Role of Molecular and Cellular Aging Pathways on Age-Related Hearing Loss.
Ege T, Tao L, North BJ. Ege T, et al. Int J Mol Sci. 2024 Sep 7;25(17):9705. doi: 10.3390/ijms25179705. Int J Mol Sci. 2024. PMID: 39273652 Free PMC article. Review. - Advances in the Study of Etiology and Molecular Mechanisms of Sensorineural Hearing Loss.
He C, Gai H, Zhao W, Zhang H, Lai L, Ding C, Chen L, Ding J. He C, et al. Cell Biochem Biophys. 2024 Sep;82(3):1721-1734. doi: 10.1007/s12013-024-01344-3. Epub 2024 Jun 7. Cell Biochem Biophys. 2024. PMID: 38849694 Review. - The Relevance of Autophagy within Inner Ear in Baseline Conditions and Tinnitus-Related Syndromes.
Lazzeri G, Biagioni F, Ferrucci M, Puglisi-Allegra S, Lenzi P, Busceti CL, Giannessi F, Fornai F. Lazzeri G, et al. Int J Mol Sci. 2023 Nov 23;24(23):16664. doi: 10.3390/ijms242316664. Int J Mol Sci. 2023. PMID: 38068993 Free PMC article. Review. - The cochlea is built to last a lifetime.
Savas JN. Savas JN. Hear Res. 2023 Sep 1;436:108821. doi: 10.1016/j.heares.2023.108821. Epub 2023 Jun 1. Hear Res. 2023. PMID: 37295280 Free PMC article. Review. - Mitochondrial dysfunction in hearing loss: Oxidative stress, autophagy and NLRP3 inflammasome.
Li P, Li S, Wang L, Li H, Wang Y, Liu H, Wang X, Zhu X, Liu Z, Ye F, Zhang Y. Li P, et al. Front Cell Dev Biol. 2023 Feb 20;11:1119773. doi: 10.3389/fcell.2023.1119773. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36891515 Free PMC article. Review.
References
- Mathers C, Smith A, Concha MGlobal Burden of Hearing Loss in the Year 2000. Global Burden of Disease: Geneva, Switzerland, pp 1–30, 2003.
- World Health Organizaton. Deafness and hearing loss. Available at http://www.who.int/mediacentre/factsheets/fs300/en.
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci 2006; 7: 837–849. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials