IL-6 receptor blockade corrects defects of XIAP-deficient regulatory T cells - PubMed (original) (raw)
IL-6 receptor blockade corrects defects of XIAP-deficient regulatory T cells
Wan-Chen Hsieh et al. Nat Commun. 2018.
Abstract
X-linked lymphoproliferative syndrome type-2 (XLP-2) is a primary immunodeficiency disease attributed to XIAP mutation and is triggered by infection. Here, we show that mouse Xiap-/- regulatory T (Treg) cells and human XIAP-deficient Treg cells are defective in suppressive function. The Xiap-/- Treg cell defect is linked partly to decreased SOCS1 expression. XIAP binds SOCS1 and promotes SOCS1 stabilization. Foxp3 stability is reduced in Xiap-/- Treg cells. In addition, Xiap-/- Treg cells are prone to IFN-γ secretion. Transfer of wild-type Treg cells partly rescues infection-induced inflammation in Xiap-/- mice. Notably, inflammation-induced reprogramming of Xiap-/- Treg cells can be prevented by blockade of the IL-6 receptor (IL-6R), and a combination of anti-IL-6R and Xiap-/- Treg cells confers survival to inflammatory infection in Xiap-/- mice. Our results suggest that XLP-2 can be corrected by combination treatment with autologous iTreg (induced Treg) cells and anti-IL-6R antibody, bypassing the necessity to transduce Treg cells with XIAP.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Fig. 1
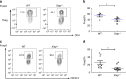
XIAP-deficiency impairs Treg cell suppressive function. a Comparable control and _Xiap_−/− tTreg cells and iTreg cells development. The fractions of splenic CD4+Foxp3+ population from control (WT) and _Xiap_−/− mice were determined (tTreg). WT and _Xiap_−/− CD4+CD25− T cells were treated with anti-CD3/CD28 plus TGF-β and IL-2, and Foxp3 expression at day 5 was assessed (iTreg). Experiments were independently repeated six times. b XIAP-deficiency does not affect the Treg cells phenotype. Expressions of CTLA-4, GITR, LAG3 and FR4 in WT and _Xiap_−/− tTreg cells were determined. Numbers indicate mean fluorescence intensities. c Normal IL-10 and TGF-β production in _Xiap_−/− tTreg cells. CD4+CD25+ cells were stimulated with anti-CD3/CD28 and IL-2 for 96 h, before generation of IL-10 and TGF-β was determined. d Impaired in vitro suppressive activity of _Xiap_−/− tTreg cells. CD4+CD25- cells were incubated with anti-CD3, antigen-presenting cells, and the indicated ratios of WT and _Xiap_−/− CD4+CD25+ tTreg cells. [3H]thymidine incorporation was determined at 80 h. Values are mean ± SD of triplicate samples in an experiment. *P < 0.05, **P < 0.01 for unpaired _t_-test. Experiments were reproduced independently three times with similar outcomes. e Knockdown of XIAP in human Treg cells. The levels of XIAP in control (pLKO.3) and XIAP-knockdown human iTreg cells were determined by immunoblots. f Impaired suppressive activity in XIAP-deficient human tTreg cells. Human effector T cells (Teff) were labeled with 2 μM CFSE, activated by anti-CD3/CD28 in the presence of the indicated ratio of human control and XIAP-knockdown tTreg cells, and collected at 72 h. Proliferation was determined by gating CD4+ T cells for their CFSE intensity in flow cytometry. g, h Diminished inhibitory activity of _Xiap_−/− tTreg cells in vivo. CD4+CD25− effector T cells were administered intraperitoneally into _Rag1_−/− mice with WT or _Xiap_−/− tTreg cells. Body weight was determined at the indicated time-points (g). Data are the mean ± SD of six mice in each group. ***P < 0.001 for two-way ANOVA. Mice were killed at day 27 and colons were removed, fixed in paraformaldehyde, sectioned, and stained with H&E. Micrographs are representative of the six mice in each group. Bar indicates 100 μm
Fig. 2
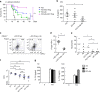
Foxp3 instability in activated _Xiap_−/− Treg cells. a, b Reduced Foxp3+ population in activated _Xiap_−/− tTreg cells. WT and _Xiap_−/− tTreg cells were stimulated with anti-CD3/CD28 in the presence of IL-2 for 4 days. Foxp3 expression of activated tTreg cells was determined by intracellular staining and analyzed by flow cytometry. The gated section represents the Foxp3+ population and the number indicates the percentage of each population (a). The Foxp3+ fractions in WT and _Xiap_−/− tTreg cells were quantitated (b), n = 5. c, d Diminished Foxp3high population in adoptively transferred _Xiap_−/− tTreg cells. CD45.2+ tTreg cells were recovered from CD45.1+ _Rag1_−/− mice into which CD45.2+ tTreg cells and CD45.1+ CD4+CD25− T cells had been transferred a month earlier. The isolated CD45.2+ CD4+ T cells were reactivated with TPA/A23187 and Foxp3 expression was determined by intracellular staining (c). The Foxp3+ fractions in the transferred WT and _Xiap_−/− tTreg cells re-isolated from _Rag1_−/− mice were quantitated (d), n = 9. *P < 0.05 for unpaired t-test
Fig. 3
XIAP interacts with SOCS1 and enhances SOCS1 expression. a XIAP deficiency impairs IL-2-induced SOCS1 expression. T cells were collected at 0, 10, 20, 40, 60, and 120 min after IL-2 treatment. SOCS1 expression of lysate was detected by anti-SOCS1. Protein levels were quantitated by densitometry and normalized by actin control. The level of SOCS1 in WT T cells was used as 1 for comparison. b XIAP-deficiency decreases SOCS1 expression in human iTreg cells. Control and XIAP-knockdown human iTreg cells were treated with IL-2 and the levels of SOCS1 were determined at the indicated time-points. c XIAP enhances SOCS1 expression. XIAP-FLAG and SOCS1-HA were co-transfected into HEK293T cells. After 24 h of transfection, cell lysates were prepared and SOCS1 and XIAP expression was determined with anti-FLAG and anti-HA. d XIAP interacts with SOCS1. XIAP-FLAG and SOCS1-HA were co-transfected into HEK293T cells as indicated. Total cell lysates were immunoprecipitated by anti-HA and the presence of SOCS1 and XIAP-FLAG in the precipitates and lysates was determined. * indicates immunoglobulin heavy chain. e Endogenous XIAP interacts with SOCS1. Mouse peripheral T cells from spleen and lymph nodes were treated with IL-2 as indicated and then 600 μg of cell lysates were immunoprecipitated with anti-SOCS1 or control goat IgG. The contents of endogenous XIAP were determined. * indicates immunoglobulin heavy chain. f The BIR1 domain of XIAP interacts with SOCS1. Full-length (FL), RING domain-deleted (ΔR), N-terminus (N), C-terminus (C), BIR1, BIR2 or BIR3 of XIAP-FLAG were co-transfected with SOCS1-HA into HEK293T cells. Total cell lysates were immunoprecipitated with anti-HA and the presence of XIAP variants and SOCS1 in the pull-down complex and cell lysates was determined. g The SH2 domain of SOCS1 binds XIAP. Full-length (FL), SOSC box-deleted (ΔSB), N-terminal-deleted (ΔN), N-terminal (N), SH2 domain, or SOCS box (SB) of SOCS1 were transfected with XIAP-FLAG into HEK293T cells as indicated. Total cell lysates were immunoprecipitated by anti-FLAG and the presence of SOCS1 variants and XIAP in the precipitates and cell lysates was determined. Each experiment (a, c–g) was independently repeated three times with similar results
Fig. 4
XIAP enhances the association of Elongin B/C with SOCS1 and promotes SOCS1 K63 ubiquitination. a XIAP increases the association of Elongin B/C with SOCS1. Full-length, ΔRING (ΔR) or C-terminal (C) XIAP-FLAG was co-transfected with SOCS1-HA and Elongin B/C-Myc into HEK293T cells. Elongin B/C-Myc in cell lysates was pulled down by anti-Myc and the presence of SOCS1 and XIAP in the precipitates and lysates was determined by the respective antibodies. b XIAP enhances the addition of K63 ubiquitin to SOCS1 in vivo. XIAP-FLAG was co-transfected with SOCS1-myc and Elongin B/C-Myc into HEK293T cells, with WT Ub, K48 Ub or K63 Ub, as indicated. SOCS1 was immune-precipitated, and the association of ubiquitin type determined. c XIAP promotes SOCS1 K63 poly-ubiquitination in vitro. Recombinant K63 ubiquitin-HA, E1, E2 (UBC13), SOCS1-HA (on Mag beads), Elongin B/C-Myc, or XIAP-FLAG was added as indicated to a ubiquitination reaction conducted at 30 °C for 1 h. Around 10% of the reaction mixture was used for Western blotting. SOCS1-HA was pulled down from the rest of the reaction mixture and ubiquitination of the SOCS1 complex was determined. Experiments were independently repeated three times (a, c) or twice (b)
Fig. 5
Enhanced conversion of XIAP-deficient Treg cells into Foxp3+IFN-γ+ cells. a, b Increased transition of _Xiap_−/− tTreg cells into IFN-γ-producing cells. WT and _Xiap_−/− tTreg cells (Foxp3-GFP tagged) were isolated by GFP-expression and re-stimulated with plate-bound anti-CD3/CD28 and IL-2 (CD3/CD28), with additional IL-12 ( + IL-12, 50 ng ml−1), for 4 days. tTreg cells were then reactivated with TPA/A23187 for 6 h, and the expressions of IFN-γ in the gated GFP+ (representing Foxp3+) fraction were determined by intracellular staining (a). Quantitation of Foxp3+IFN-γ+ cells from tTreg cells isolated by either Foxp3-GFP or CD4+CD25+ expression (b), n = 10. ***P < 0.001 for unpaired _t_-test. c Increased secretion of IFN-γ by _Xiap_−/− tTreg cells. Purified WT and _Xiap_−/− tTreg cells were stimulated as in (a), or with additional IL-6 plus IL-1α + IL-1β ( + IL-6/IL-1), and re-activated with TPA/A23187 for 24 h and the levels of IFN-γ in supernatants was analyzed by ELISA. d, e Enhanced expression and secretion of IFN-γ by human XIAP-knockdown iTreg cells. Human control and XIAP-knockdown iTreg cells were activated by anti-CD3/CD28 and IL-2, with the addition of IL-12 or IL-6, as indicated, for 4 days. Secretion of IFN-γ was quantitated (d) and intracellular expressions of Foxp3 and IFN-γ were determined (e). f Elevated secretion of IL-17A by _Xiap_−/− tTreg cells. WT and _Xiap_−/− tTreg cells were stimulated as in (c) and the generated IL-17 was quantitated by ELISA. g, h Increased expression of IFN-γ and IL-17 in the adoptively transferred _Xiap_−/− tTreg cells. WT or _Xiap_−/− CD45.2+ tTreg cells were administrated into _Rag1_−/− CD45.1+ mice together with WT CD45.1+ CD4+CD25− effector T cells. Mice were killed after 27 days and CD4+ T cells from spleen and lymph nodes were isolated. CD4+ T cells were activated by TPA/A23187 and the expressions of Foxp3, IFN-γ (g) and IL-17A (h) in CD45.2+ cells were determined by intracellular staining. Values (c, e, f) are mean ± SD of triplicate samples in an experiment. *P < 0.05, **P < 0.01, ***P < 0.001 for unpaired t-test. All experiments (a–h) were independently repeated three times with similar results
Fig. 6
Transfer of Treg cells rescues _Xiap_−/− mice from lethal Candida albicans infection. a Increased expression of IFN-γ+ and IL-17+ cells in _Xiap_−/− Treg cells upon Candida albicans infection. WT and _Xiap_−/− female mice were intravenously infected with C. albicans (1 × 105), and mice were killed at day 10. T cells were isolated from spleen, and the frequencies of CD4+Foxp3+, Foxp3+IFN-γ+ and Foxp3+IL-17+ cells were quantitated by intracellular staining. b Transfer of WT iTreg cells partially rescues _Xiap_−/− mice from _C. albicans_-induced lethality. WT and _Xiap_−/− female mice were intravenously administered with C. albicans (1 × 105) and a group of _Xiap_−/− mice also received WT iTreg cells (1 × 106) at day 2. Survival of mice was monitored and is presented as a Kaplan–Meier survival curve (n = 7 for each group). **P < 0.01. c Reduced kidney inflammation in _C. albicans_-infected _Xiap_−/− mice with WT iTreg cells transfer. Kidney was isolated from _Xiap_−/− mice 10 days after C. albicans injection and infiltrated neutrophil contents were determined. d Reduced inflammatory cytokine production in _C. albicans_-infected _Xiap_−/− mice into which WT iTreg cells had been transferred. Serum from mice was collected at the indicated time-points after C. albicans injection, and the levels of IL-6, TNF, MCP-1, and IL-12 were determined, n = 6. Values (a, d) are mean ± SD of samples. *P < 0.05, **P < 0.01, ***P < 0.001 for unpaired _t_-test
Fig. 7
Anti-IL-6R reduces the expression of IFN-γ in activated _Xiap_−/− Treg cells. a, b Anti-IL-6R decreases IFN-γ expression in re-stimulated _Xiap_−/− Treg cells. WT and _Xiap_−/− iTreg cells were stimulated with anti-CD3/CD28 and IL-2, or with additional IL-12 as indicated, in the absence or presence of anti-IL-6R (50 μg ml−1) for 4 days. iTreg cells were re-stimulated with TPA/A23187 for 24 h and IFN-γ production was determined by ELISA (a), or reactivated with TPA/A23187 for 5 h and the expressions of Foxp3 and IFN-γ were determined by intracellular staining (b). c, d Anti-IL-6R inhibits IFN-γ expression in human Treg cells. Control and human XIAP-knockdown iTreg cells were stimulated as in (a, b), with additional IL-6 as indicated, and secretion (c) or intracellular expression (d) of IFN-γ was determined. e Inability of anti-TNF or anti-IL-1R to inhibit the production of IFN-γ in activated _Xiap_−/− Treg cells. WT and _Xiap_−/− iTreg cells were stimulated, as described in (a), in the presence or absence of anti-TNF or anti-IL-1R (50 μg ml−1 each) for 4 days. Treg cells were re-stimulated with TPA/A23187 for 24 h and the secreted IFN-γ was determined by ELISA. f Anti-IL-6R rescues the impaired suppressive activity of _Xiap_−/− tTreg cells in vivo. CD45.2+ WT or _Xiap_−/− tTreg cells were co-transferred with CD45.1+ CD4+CD25- effector T cells into male CD45.1+ _RagI_−/− mice. Anti-IL-6R antibody (500 μg per mouse) was intraperitoneally administrated at day 0, followed by weekly dosing of 500 μg. The body weights of mice were monitored at the indicated time-points. ***P < 0.001 by two-way ANOVA. g, h Anti-IL-6R restores Foxp3 stability and reduces IFN-γ expression in _Xiap_−/− tTreg cells transferred in vivo. Lymphocytes were isolated from spleen and mesenteric lymph nodes of mice in (f) and reactivated with TPA/A23187. The expressions of Foxp3 (g) and IFN-γ (h) in CD45.2+ T cells were determined by intracellular staining. Values are mean ± SD of triplicate samples in an experiment. *P < 0.05, **P < 0.01, ***P < 0.001 for unpaired _t_-test (a, c, g, h). Experiments (a–e) were independently repeated three times with similar results
Fig. 8
Combination anti-IL-6R and _Xiap_−/− Treg cells rescue mice from infection-induced inflammation. a, b _Xiap_−/− iTreg cells plus anti-IL-6R rescue _Xiap_−/− mice from C. albicans infection. Male _Xiap_−/− mice (KO) were intravenously administered with C. albicans and _Xiap_−/− iTreg cells (1 × 106) or anti-IL-6R antibody (500 μg) at day 2, or both, as indicated. Survival of mice is presented as a Kaplan–Meier survival curve (a). n = 11 for KO, n = 10 for KO + KO iTreg, n = 6 for KO + Ab, n = 9 for KO + KO iTreg + Ab. ***P < 0.001 for Log-rank (Mantel–Cox) Test (a). n.s., not significant. The serum level of IL-6 was determined on day 14 after infection (b). *P < 0.05 for unpaired _t_-test (b). c, d Anti-IL-6R prevents the conversion of the transferred _Xiap_−/− Treg cells. CD45.1+ _Xiap_−/− iTreg cells were transferred into CD45.2+ _Xiap_−/− mice infected with C. albicans as in (a). CD45.1+ T cells were recovered at day 10 post-infection, and the expression of Foxp3 and IFN-γ was determined (c). The Foxp3+IFN-γ+ fractions in the CD45.1+ Treg cells were quantitated (d), n = 5. **P < 0.01 for unpaired _t_-test (d). e, f Anti-IL-6R reduces kidney neutrophil infiltration and fungal load in _C. albicans_-infected _Xiap_−/− mice with KO iTreg cells transfer. Male _Xiap_−/− mice were infected with C. albicans, as described in (a). Kidneys were isolated 14 days post-infection and neutrophils (e) and C. albicans titres (f) were quantitated. *P < 0.05, **P < 0.01 for unpaired _t_-test. n.s., not significant. g Anti-IL-6R does not affect IL-17 production. Male CD45.1+ _Xiap_−/− mice were infected with C. albicans and treated with anti-IL-6R and anti-IL-6R plus CD45.2+ KO iTreg cells. Spleen T cells and B cells were isolated 7 days after treatments. CD45.2+ iTreg cells were depleted. T cells were incubated with B cells and heat-killed C. albicans (HKCA) for 4 days, and the production of IL-17A after stimulation with TPA/A23187 (10/100 ng ml−1) was determined by ELISA, . Values are mean ± SD of triplicate samples in an experiment. Experiments were independently repeated three times with similar results
Similar articles
- Intact Regulatory T-Cell Function but Defective Generation of IL-17A-Producing CD4+ T Cells in XIAP Deficiency.
Gurram B, Hammelev E, Syverson G, Haribhai D, Yan K, Simpson P, Salzman N, Verbsky JW. Gurram B, et al. J Pediatr Gastroenterol Nutr. 2016 Aug;63(2):218-25. doi: 10.1097/MPG.0000000000001122. J Pediatr Gastroenterol Nutr. 2016. PMID: 26825770 - Sustained elevation of serum interleukin-18 and its association with hemophagocytic lymphohistiocytosis in XIAP deficiency.
Wada T, Kanegane H, Ohta K, Katoh F, Imamura T, Nakazawa Y, Miyashita R, Hara J, Hamamoto K, Yang X, Filipovich AH, Marsh RA, Yachie A. Wada T, et al. Cytokine. 2014 Jan;65(1):74-8. doi: 10.1016/j.cyto.2013.09.007. Epub 2013 Sep 29. Cytokine. 2014. PMID: 24084330 - XIAP deficiency syndrome in humans.
Latour S, Aguilar C. Latour S, et al. Semin Cell Dev Biol. 2015 Mar;39:115-23. doi: 10.1016/j.semcdb.2015.01.015. Epub 2015 Feb 7. Semin Cell Dev Biol. 2015. PMID: 25666262 Review. - XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome.
Rigaud S, Fondanèche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, de Saint Basile G, Latour S. Rigaud S, et al. Nature. 2006 Nov 2;444(7115):110-4. doi: 10.1038/nature05257. Nature. 2006. PMID: 17080092 - The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases.
Mohr A, Atif M, Balderas R, Gorochov G, Miyara M. Mohr A, et al. Clin Exp Immunol. 2019 Jul;197(1):24-35. doi: 10.1111/cei.13288. Epub 2019 Mar 24. Clin Exp Immunol. 2019. PMID: 30830965 Free PMC article. Review.
Cited by
- Retinoic acid signaling acts as a rheostat to balance Treg function.
Thangavelu G, Andrejeva G, Bolivar-Wagers S, Jin S, Zaiken MC, Loschi M, Aguilar EG, Furlan SN, Brown CC, Lee YC, Hyman CM, Feser CJ, Panoskaltsis-Mortari A, Hippen KL, MacDonald KP, Murphy WJ, Maillard I, Hill GR, Munn DH, Zeiser R, Kean LS, Rathmell JC, Chi H, Noelle RJ, Blazar BR. Thangavelu G, et al. Cell Mol Immunol. 2022 Jul;19(7):820-833. doi: 10.1038/s41423-022-00869-y. Epub 2022 May 17. Cell Mol Immunol. 2022. PMID: 35581350 Free PMC article. - Infiltrating treg reprogramming in the tumor immune microenvironment and its optimization for immunotherapy.
Zhou Z, Xu J, Liu S, Lv Y, Zhang R, Zhou X, Zhang Y, Weng S, Xu H, Ba Y, Zuo A, Han X, Liu Z. Zhou Z, et al. Biomark Res. 2024 Sep 4;12(1):97. doi: 10.1186/s40364-024-00630-9. Biomark Res. 2024. PMID: 39227959 Free PMC article. Review. - Infection-induced inflammation from specific inborn errors of immunity to COVID-19.
Ku CL, Chen IT, Lai MZ. Ku CL, et al. FEBS J. 2021 Sep;288(17):5021-5041. doi: 10.1111/febs.15961. Epub 2021 May 20. FEBS J. 2021. PMID: 33971084 Free PMC article. Review. - Treg in inborn errors of immunity: gaps, knowns and future perspectives.
Kennedy-Batalla R, Acevedo D, Luo Y, Esteve-Solé A, Vlagea A, Correa-Rocha R, Seoane-Reula ME, Alsina L. Kennedy-Batalla R, et al. Front Immunol. 2024 Jan 8;14:1278759. doi: 10.3389/fimmu.2023.1278759. eCollection 2023. Front Immunol. 2024. PMID: 38259469 Free PMC article. Review. - Immunity to X-linked inhibitor of apoptosis protein (XIAP) in malignant melanoma and check-point blockade.
Zhou J, Li J, Guleria I, Chen T, Giobbie-Hurder A, Stevens J, Gupta M, Wu X, Brennick RC, Manos MP, Hodi FS. Zhou J, et al. Cancer Immunol Immunother. 2019 Aug;68(8):1331-1340. doi: 10.1007/s00262-019-02370-4. Epub 2019 Jul 18. Cancer Immunol Immunother. 2019. PMID: 31317218 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials