Myristoylated methionine sulfoxide reductase A is a late endosomal protein - PubMed (original) (raw)
Myristoylated methionine sulfoxide reductase A is a late endosomal protein
Jung Mi Lim et al. J Biol Chem. 2018.
Abstract
Methionine residues in proteins provide antioxidant defense by reacting with oxidizing species, which oxidize methionine to methionine sulfoxide. Reduction of the sulfoxide back to methionine is catalyzed by methionine sulfoxide reductases, essential for protection against oxidative stress. The nonmyristoylated form of methionine sulfoxide reductase A (MSRA) is present in mitochondria, whereas the myristoylated form has been previously reported to be cytosolic. Despite the importance of MSRA in antioxidant defense, its in vivo binding partners and substrates have not been identified. Starting with a protein array, and followed by immunoprecipitation experiments, colocalization studies, and subcellular fractionation, we identified the late endosomal protein, StAR-related lipid transfer domain-containing 3 (STARD3), as a binding partner of myristoylated MSRA, but not of nonmyristoylated MSRA. STARD3 is known to have both membrane-binding and cytosolic domains that are important in STARD3-mediated transport of cholesterol from the endoplasmic reticulum to the endosome. We found that the STARD3 cytosolic domain localizes MSRA to the late endosome. We propose that the previous conclusion that myristoylated MSRA is strictly a cytosolic protein is artifactual and likely due to vigorous overexpression of MSRA. We conclude that myristoylated MSRA is a late endosomal protein that may play a role in lipid metabolism or may protect endosomal proteins from oxidative damage.
Keywords: STARD3; endosome; methionine; methionine sulfoxide reductase A; oxidation–reduction (redox); oxidative stress; protein myristoylation.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Figure 1.
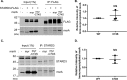
Myristoylated MSRA interacts with STARD3. A, HEK293 cells coexpressing FLAG-tagged STARD3 and either the WT or active site mutant (C74S) of myristoylated MSRA were immunoprecipitated with anti-FLAG antibody and probed with anti-MSRA antibody. Both the WT and active site mutant strongly interact with STARD3. The asterisk marks the IgG heavy chain. The molecular size markers of protein are indicated in kDa. B, quantitation of 3 replicates of the experiment shown in panel A, run on separate days. The fluorescence intensity of the WT was set to 1.0. The mean ± S.D. are shown as lines. NS, not significantly different from the WT with a paired two-tailed t test, p = 0.45. C, myristoylated MsrA alone was transfected in HEK293 cells. Endogenous STARD3 was immunoprecipitated with mouse anti-STARD3 antibody and detected by an anti-STARD3 antibody. MSRA was detected by an anti-MSRA antibody. The asterisk marks a nonspecific band. myr, myristoylated. D, quantitation of 3 replicates of the experiment shown in panel C, run on separate days. The fluorescence intensity of the WT was set to 1.0. The mean ± S.D. are shown as lines. NS, not significantly different from the WT with a paired two-tailed t test, p = 0.67.
Figure 2.
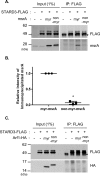
STARD3 interacts only with myristoylated MSRA. A, FLAG-tagged STARD3 was coexpressed in HEK293 cells with myristoylated or nonmyristoylated MSRA. Cell lysates were immunoprecipitated with anti-FLAG antibody and probed with an anti-MSRA antibody. The asterisk marks the IgG heavy chain. B, quantitation of 3 replicates of the experiment shown in panel A, run on separate days. The fluorescence intensity of the myristoylated MSRA was set to 1.0. The mean ± S.D. are shown as lines. *, p < 0.0001 with the paired two-tailed t test. C, myristoylated or nonmyristoylated ARF1, tagged with HA at its C terminus, was overexpressed with FLAG-tagged STARD3 in HEK293 cells. Cell lysates were immunoprecipitated with anti-FLAG antibody and detected by anti-HA antibody. The asterisk marks the heavy chain of IgG. This experiment was performed 3 times on separate days, none of which detected an interaction between ARF1 and STARD3.
Figure 3.
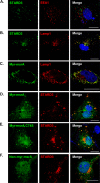
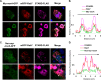
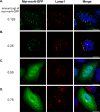
Myristoylated MSRA is colocalized with STARD3 at the late endosome. A and B, COS7 cells expressing FLAG-tagged STARD3 were stained with anti-EEA1, an early endosome marker (A) and anti-LAMP1, a late endosome marker (B). C, myristoylated MsrA was transiently transfected in COS7 cells, which were stained with anti-MSRA and anti-LAMP1. D–F, MsrA and FLAG-tagged Stard3 were transiently transfected in COS7 cells, which were stained with anti-MSRA and anti-FLAG. D, the myristoylated form of MSRA was expressed. E, the inactive form of myristoylated MSRA (C74S) was expressed. F, the nonmyristoylated form of MSRA was expressed. Scale bars, 20 μm.
Figure 4.
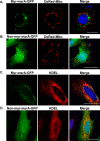
Neither myristoylated MSRA nor nonmyristoylated MSRA is localized to mitochondria or the endoplasmic reticulum. A and B, COS7 cells were cotransfected with either myristoylated MsrA-gfp (A) or nonmyristoylated MsrA (B) and DsRed-Mito, a mitochondrial marker protein. C and D, HeLa cells expressing either myristoylated MSRA-GFP (C) or nonmyristoylated MSRA (D) were stained with anti-KDEL, an endoplasmic reticulum marker protein. Scale bars, 20 μm.
Figure 5.
Myristoylated MSRA is anchored to the membrane of late endosomes. A and C, COS7 cells were transiently transfected with three constructs, Stard3-FLAG, mRFP-Rab7, a late endosomal protein, and either (A) myristoylated _MsrA_-gfp or (C) nonmyristoylated MsrA-gfp. After 8 h, cells were treated with 20 m
m
NH4Cl for 16 h to alkalinize and enlarge late endosomes and lysosomes. The images within the white box of the upper panels are magnified in the lower panels. Scale bar, 10 μm. B and D, the merged images were scanned along the white arrow for relative fluorescence intensity with ZEN 2 software. The black arrows indicate the direction of scanning.
Figure 6.
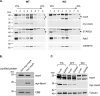
Subcellular fractionation demonstrates that myristoylated MSRA is enriched in the late endosome fraction. A, myristoylated MSRA is most prominent in fraction 1 from wildtype (WT) mouse liver and is not present in fractions prepared from the MSRA knockout liver (KO). Fraction 1 also contains most of the intact late endosomes. Each fraction was evaluated by immunoblot with these marker proteins: RAB7 (late endosome), mtHSP70 (mitochondria), myristoylated MSRA, total MSRA, and STARD3. The asterisk (*) indicates a nonspecific band. The molecular size markers of protein are indicated in kDa. The specificity of the mAb for the myristoylated form of MSRA is shown in panel B. 100 ng of purified, recombinant human myristoylated or nonmyristoylated MSRA were subjected to SDS-PAGE followed by immunoblotting by either the anti-myristoylated MSRA antibody or the general anti-MSRA antibody. As indicated under “Experimental procedures,” the concentration of protein was determined spectrophotometrically. However, to assure that equal amounts of the two proteins were loaded for immunoblotting, we also subjected 1 μg of each protein SDS-PAGE followed by staining with Coomassie Brilliant Blue. C, the anti-myristoylated MSRA and general anti-MSRA antibodies are specific for MSRA. This was demonstrated by immunoblotting homogenates of liver and kidney from WT and MSRA knockout mice. The asterisk (*) marks nonspecific bands detected by the general anti-MSRA antibody. Tubulin served as a loading control.
Figure 7.
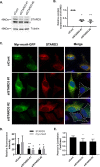
Knocking down STARD3 decreases MSRA on late endosomes. A, HeLa cells were transfected for 48 h with nontargeting siRNA (siCont) or 2 different Stard3 targeting oligomers (siSTARD3 #1 and siSTARD3 #2). STARD3 was quantitated by immunoblotting with anti-STARD3, with tubulin serving as a loading control. The asterisk (*) marks a nonspecific band. B, quantitation of 3 replicates of the experiment shown in panel A, run on separate days. The fluorescence intensity of the STARD3 with control siRNA was set to 1.0. The mean ± S.D. are shown as lines. Compared with the control using the paired two-tailed t test, *, p < 0.0001 and **, p < 0.0003. C, 30 h after transfection with siRNA, HeLa cells were transfected with myristoylated MsrA-gfp. 18 h later they were stained with anti-STARD3 antibody. Scale bar, 20 μm. The dashed lines outline STARD3-depleted cells. 40 such depleted cells were measured for each siRNA and the results shown in panel D. D, relative fluorescence intensity of myristoylated MSRA and STARD3 was determined using ZEN 2 software (Carl Zeiss) from control and STARD3-depleted cells. 40 cells were analyzed for each sample in 3 independent experiments performed on separate days. The mean ± S.D. are shown as lines. Compared with the control using the paired two-tailed t test; *, p < 0.0001 and **, p < 0.0002. E, colocalization of myristoylated MSRA and STARD3. 40 cells were analyzed for each sample in 3 independent experiments performed on separate days, and the Pearson's correlation coefficient determined with ImageJ software. The mean ± S.D. are shown as lines. Compared with the control siRNA using the paired two-tailed t test, *, p < 0.0001.
Figure 8.
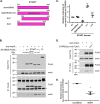
The effect of deletions and mutations on the binding of MSRA to STARD3. The rationale for each construct is given in the text. A, diagram of constructs. B, HEK293 cells co-expressing myristoylated MSRA and one of the STARD3 shown in panel A were immunoprecipitated with anti-FLAG antibody and then probed with anti-MSRA and anti-FLAG antibodies. The single and double asterisks mark the heavy and light chains of IgG. C, quantitation of 3 replicates of the experiment shown in panel A, run on separate days. The fluorescence intensity of the full-length STARD3 (FL, STARD3(1–444)) was set to 1.0. The mean ± S.D. are shown as lines. Compared with the unmodified START domain using the paired two-tailed t test: *, p = 0.003; **, p < 0.0001; ***, p = 0.18 versus unmodified START domain. D, HEK293 cells co-expressing myristoylated MSRA and either unmodified START domain or M307V were immunoprecipitated with anti-FLAG. The protein amounts were measured with anti-FLAG and anti-MSRA. E, quantitation of 2 replicates of the experiment shown in panel D, run on separate days. The fluorescence intensity of the unmodified protein was set to 1.0. The mean ± S.D. are shown as lines. The M307V mutant was compared with the unmodified protein with the two-tailed t test, *, p = 0.01.
Figure 9.
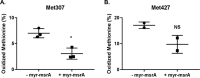
Methionine sulfoxide 307 in oxidatively modified STARD3 is reduced by MSRA. The panels plot the percentage of methionine sulfoxide before and after myristoylated msrA treatment. A, Met-307. Three preparations were made and analyzed on separate days. The mean ± S.D. are shown as lines. Methionine sulfoxide was reduced in the myristoylated MSRA incubated STARD3 compared with the control using the paired two-tailed t test; *, p = 0.008. B, Met-427. Two preparations were made and analyzed on separate days. The mean ± S.D. are shown as lines. NS, not significantly different with a paired two-tailed t test, p = 0.11.
Figure 10.
Transfection with higher amounts of DNA causes cytosolic localization of myristoylated MSRA. A–D, COS7 cells were transfected with the indicated amount of myristoylated MsrA-gfp. After 24 h, cells were stained for the late endosomal marker, LAMP1. Scale bars, 10 μm.
Similar articles
- Adenovirus Modulates Toll-Like Receptor 4 Signaling by Reprogramming ORP1L-VAP Protein Contacts for Cholesterol Transport from Endosomes to the Endoplasmic Reticulum.
Cianciola NL, Chung S, Manor D, Carlin CR. Cianciola NL, et al. J Virol. 2017 Feb 28;91(6):e01904-16. doi: 10.1128/JVI.01904-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077646 Free PMC article. - Methionine sulfoxide reductases and cholesterol transporter STARD3 constitute an efficient system for detoxification of cholesterol hydroperoxides.
Lim JM, Sabbasani VR, Swenson RE, Levine RL. Lim JM, et al. J Biol Chem. 2023 Sep;299(9):105099. doi: 10.1016/j.jbc.2023.105099. Epub 2023 Jul 26. J Biol Chem. 2023. PMID: 37507014 Free PMC article. - Management of urinary stones by experts in stone disease (ESD 2025).
Papatsoris A, Geavlete B, Radavoi GD, Alameedee M, Almusafer M, Ather MH, Budia A, Cumpanas AA, Kiremi MC, Dellis A, Elhowairis M, Galán-Llopis JA, Geavlete P, Guimerà Garcia J, Isern B, Jinga V, Lopez JM, Mainez JA, Mitsogiannis I, Mora Christian J, Moussa M, Multescu R, Oguz Acar Y, Petkova K, Piñero A, Popov E, Ramos Cebrian M, Rascu S, Siener R, Sountoulides P, Stamatelou K, Syed J, Trinchieri A. Papatsoris A, et al. Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review. - HIV-1 Nef Antagonizes SERINC5 Restriction by Downregulation of SERINC5 via the Endosome/Lysosome System.
Shi J, Xiong R, Zhou T, Su P, Zhang X, Qiu X, Li H, Li S, Yu C, Wang B, Ding C, Smithgall TE, Zheng YH. Shi J, et al. J Virol. 2018 May 14;92(11):e00196-18. doi: 10.1128/JVI.00196-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514909 Free PMC article. - Disorders of Intracellular Cobalamin Metabolism.
Sloan JL, Carrillo N, Adams D, Venditti CP. Sloan JL, et al. 2008 Feb 25 [updated 2021 Dec 16]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2008 Feb 25 [updated 2021 Dec 16]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301503 Free Books & Documents. Review.
Cited by
- The Oxidized Protein Repair Enzymes Methionine Sulfoxide Reductases and Their Roles in Protecting against Oxidative Stress, in Ageing and in Regulating Protein Function.
Lourenço Dos Santos S, Petropoulos I, Friguet B. Lourenço Dos Santos S, et al. Antioxidants (Basel). 2018 Dec 12;7(12):191. doi: 10.3390/antiox7120191. Antioxidants (Basel). 2018. PMID: 30545068 Free PMC article. Review. - Wheat gluten hydrolysates promotes fermentation performance of brewer's yeast in very high gravity worts.
Yang H, Coldea TE, Zeng Y, Zhao H. Yang H, et al. Bioresour Bioprocess. 2021 Jan 7;8(1):5. doi: 10.1186/s40643-020-00355-1. Bioresour Bioprocess. 2021. PMID: 38650257 Free PMC article. - Structure and Electron-Transfer Pathway of the Human Methionine Sulfoxide Reductase MsrB3.
Javitt G, Cao Z, Resnick E, Gabizon R, Bulleid NJ, Fass D. Javitt G, et al. Antioxid Redox Signal. 2020 Oct 1;33(10):665-678. doi: 10.1089/ars.2020.8037. Epub 2020 Aug 11. Antioxid Redox Signal. 2020. PMID: 32517586 Free PMC article. - The association of protein-bound methionine sulfoxide with proteomic basis for aging in beech seeds.
Kalemba EM, Gevaert K, Impens F, Dufour S, Czerwoniec A. Kalemba EM, et al. BMC Plant Biol. 2024 May 8;24(1):377. doi: 10.1186/s12870-024-05085-6. BMC Plant Biol. 2024. PMID: 38714916 Free PMC article.
References
- Lee B. C., Péterfi Z., Hoffmann F. W., Moore R. E., Kaya A., Avanesov A., Tarrago L., Zhou Y., Weerapana E., Fomenko D. E., Hoffmann P. R., and Gladyshev V. N. (2013) MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol. Cell 51, 397–404 10.1016/j.molcel.2013.06.019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials