3'-Sialyllactose as an inhibitor of p65 phosphorylation ameliorates the progression of experimental rheumatoid arthritis - PubMed (original) (raw)
. 2018 Dec;175(23):4295-4309.
doi: 10.1111/bph.14486. Epub 2018 Oct 17.
Li-Jung Kang 1 2 3, Kwang Min Lee 5, Chanmi Cho 1 2 3, Jae-In Lee 6, Young Bae Ryu 6, Tae Hyun Youm 4 7, Jimin Jeon 1 2 3, Mi Ra Cho 8, Seon-Yong Jeong 2 9, Sang-Rae Lee 10 11, Wook Kim 12, Siyoung Yang 1 2 3
Affiliations
- PMID: 30152858
- PMCID: PMC6240131
- DOI: 10.1111/bph.14486
3'-Sialyllactose as an inhibitor of p65 phosphorylation ameliorates the progression of experimental rheumatoid arthritis
Li-Jung Kang et al. Br J Pharmacol. 2018 Dec.
Abstract
Background and purpose: 3'-Sialyllactose (3'-SL) is a safe compound that is present in high levels in human milk. Although it has anti-inflammatory properties and supports immune homeostasis, its effect on collagen-induced arthritis (CIA) is unknown. In this study, we investigated the prophylactic and therapeutic effect of 3'-SL on the progression of rheumatoid arthritis (RA) in in vitro and in vivo models.
Experimental approach: The anti-arthritic effect of 3'-SL was analysed with fibroblast-like synoviocytes in vitro and an in vivo mouse model of CIA. RT-PCR, Western blotting and ELISA were performed to evaluate its effects in vitro. Histological analysis of ankle and knee joints of mice with CIA was performed using immunohistochemistry, as well as safranin-O and haematoxylin staining.
Key results: 3'-SL markedly alleviated the severity of CIA in the mice by reducing paw swelling, clinical scores, incidence rate, serum levels of inflammatory cytokines and autoantibody production. Moreover, 3'-SL reduced synovitis and pannus formation and suppressed cartilage destruction by blocking secretion of chemokines, pro-inflammatory cytokines, matrix metalloproteinases and osteoclastogenesis via NF-κB signalling. Notably, phosphorylation of p65, which is a key protein in the NF-κB signalling pathway, was totally blocked by 3'-SL in the RA models.
Conclusions and implications: 3'-SL ameliorated pathogenesis of CIA by suppressing catabolic factor expression, proliferation of inflammatory immune cells and osteoclastogenesis. These effects were mediated via blockade of the NF-κB signalling pathway. Therefore, 3'-SL exerted prophylactic and therapeutic effects and could be a novel therapeutic agent for the treatment of RA.
© 2018 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures
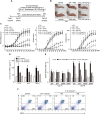
Figure 1
Prophylactic effect of 3′‐SL in CIA. (A) Experimental scheme for the analysis of CIA. DBA/1 mice were immunized on days 0 and 21 and were administered 3′‐SL at 2‐day intervals for 4 weeks after the second immunization. (B) Representative hind paws from each treatment group (4 weeks after induction of arthritis). (C) Paw swelling (left), clinical score (middle) and incidence (right) in mice with CIA that were administrated PBS, lactose, sialic acid or 3′‐SL. (D, E) Production of pro‐inflammatory cytokines (D) and CII‐specific autoantibodies (E) in the sera of mice with CIA that were administered lactose or 3′‐SL. Serum samples were collected on day 21 or 48 from mice with CIA and those administered 3′‐SL. Production of autoantibodies and cytokines was measured by ELISA. Values are presented as means ± SEM (n = 8 mice per group). (F) Percentage of CD19+B220+ B cells in the spleens from CIA mice treated with lactose or 3′‐SL. # P < 0.05, significantly different from control group; *P < 0.05, significantly different from CIA group (given lactose).
Figure 2
Amelioration of CIA severity by oral 3′‐SL via suppression of CD90.2+ FLS, macrophages and neutrophils. CIA severity was assessed by histological analysis. (A–D) Levels of infiltrated mononuclear cells into synovium of ankle (A) and knee (C) joints were analysed by haematoxylin and safranin‐O staining. Quantification of synovitis (B and D; upper panels) and pannus formation (B and D; lower panels) in ankle (B) and knee (D) joints was assessed using clinical scores. (E–G) Increased CD90.2+ FLS, macrophages and neutrophils were stained with anti‐CD90.2, ‐CD68 and ‐elastase in synovial tissues respectively. Scale bar = 100 μm. (H–J) Quantification of increased FLS (H), macrophages (I) and neutrophils (J) in ankle (left) and knee (right) joints. Numbers of increased FLS, macrophages and neutrophils were quantified by ImageJ software v1.60. Values are presented as means ± SEM (n = 8 mice per group). # P < 0.05, significantly different from control group; *P < 0.05, significantly different from CIA group (given lactose).
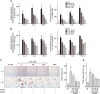
Figure 3
Effect of 3′‐SL on expression of chemokines and pro‐inflammatory cytokines in human RA‐FLS and mouse CD90.2+ FLS. (A) Human RA‐FLS and mouse CD90.2+ FLS were treated with IL‐1β (1 ng·mL−1) for the indicated times. Chemokine (CCL2, CCL5, CCL7, CXCL1, CXCL2 and CXCL5), pro‐inflammatory cytokine (IL‐1β, IL‐6 and TNF‐α) and COX2 mRNA were detected by RT‐PCR. (B) Human RA‐FLS and mouse CD90.2+ FLS were treated with IL‐1β (1 ng·mL−1) with or without various concentrations of 3′‐SL for 24 h. RT‐PCR was carried out to evaluate expression of chemokines, pro‐inflammatory cytokines and COX2. (C) PGE2 production was detected in human RA‐FLS (upper panel) and mouse CD90.2+ FLS (lower panel) after treatment with IL‐1β and 3′‐SL. (D) Immunohistochemical staining of representative chemokines, pro‐inflammatory cytokines and COX2 in the synovial tissues of ankle (left) and knee (right) joints. Scale bar = 50 μm. (E) Quantification of CCL2, CXCL1, IL‐6 and COX2 expression in the ankle (left) and knee (right) joints. Values are presented as means ± SEM (in vivo, 8 mice per group; in vitro, n = 5). # P < 0.05, significantly different from control group; *P < 0.05, significantly different from IL‐1β‐treated group or CIA group (given lactose).
Figure 4
The effects of 3′‐SL on the activation of T cells, monocytes and osteoclastogenesis. (A) Human Jurkat T cells and (B) Human THP‐1 monocytes cultured with PMA/A23187 (P/A) and LPS, respectively, were treated with or without various concentrations of 3′‐SL and MTX for 24 h. The transcript and protein levels of IL‐1β, IL‐6 and TNF‐α were determined by qRT‐PCR (A, B; left panel) and ELISA (A, B; right panel). (C) Osteoclast precursor cells were stimulated with RANKL and co‐treated with different concentrations of 3′‐SL and MTX for 6 days. The cells were stained using TRAP staining solution and photographed under light microscopy. (×40; left panel) and TRAP‐positive osteoclasts were counted (right panel). (D) TRAP activity was measured by absorbance at 405 nm using an ELISA microplate reader. Values are presented as means ± SEM (n = 5). # P < 0.05, significantly different from control group; *P < 0.05, significantly different from stimulated group.
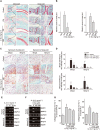
Figure 5
Protective effect of 3′‐SL against cartilage destruction in CIA development via suppression of MMP expression. (A) Mice with CIA were treated with 3′‐SL at 2 day intervals for 4 weeks with lactose or 3′‐SL, given by gavage. Cartilage destruction in ankle and knee joints was detected by haematoxylin and safranin‐O staining. (B) Cartilage destruction in ankle (left panel) and knee (right panel) joints was quantified using Mankin scores. (C) Immunohistochemical staining of MMP3 and MMP13 in synovial tissues of ankle and knee joints of mice that were administered lactose or 3′‐SL. Scale bar = 100 μm. (D) Quantification of MMP3 and MMP13 in ankle (upper panel) and knee (lower panel) joints. (E) Human RA‐FLS (upper panel) and mouse CD90.2+ FLS (lower panel) were treated with IL‐1β (1 ng·mL−1) for the indicated times. Transcript levels of MMP3 and MMP13 were detected by RT‐PCR. (F) Inhibition of IL‐1β‐induced MMP3, MMP13 and COX2 expression in human RA‐FLS (upper panel) and mouse CD90.2+ FLS (lower panel) by 3′‐SL. CD90.2+ FLS were treated with IL‐1β (1 ng·mL−1) with or without various concentrations of 3′‐SL for 24 h. The expression of MMP3, MMP13 and COX2 was determined by RT‐PCR. GAPDH was used as loading control. (G) Collagenase activity was detected in human RA‐FLS (left) and mouse CD90.2+ FLS (right) treated with IL‐1β and 3′‐SL. Values are presented as means ± SEM (in vivo, 8 mice per group; in vitro, n = 5). # P < 0.05, significantly different from control group; *P < 0.05, significantly different from CIA group (given lactose) or IL‐1β‐treated group.
Figure 6
Blocking NF‐κB activity by 3′‐SL in RA models in vitro and in vivo. (A) Human RA‐FLS and mouse CD90.2+ FLS transfected with NF‐κB reporter gene constructs and β‐galactosidase vector were treated with IL‐1β (1 ng·mL−1) with or without 3′‐SL. NF‐κB transcriptional activity was determined by reporter gene assay. (B) Human RA‐FLS and mouse CD90.2+ FLS were co‐treated with different concentrations of 3′‐SL for 24 h with IL‐1β (1 ng·mL−1). Phosphorylation of p65 was detected by Western blots. (C) Immunohistochemical staining for pp65 in synovial tissues of ankle and knee joints after oral administration of 3′‐SL to mice with CIA. Scale bar = 50 μm. (D) Quantification of pp65 expression in ankle (upper panel) and knee (lower panel) joints. Values are presented as means ± SEM (in vivo, 8 mice per group; in vitro, n = 5). # P < 0.05, significantly different from control; *P < 0.05, significantly different from IL‐1β‐treated group or CIA group (given lactose).
Similar articles
- Polyphyllin I Ameliorates Collagen-Induced Arthritis by Suppressing the Inflammation Response in Macrophages Through the NF-κB Pathway.
Wang Q, Zhou X, Zhao Y, Xiao J, Lu Y, Shi Q, Wang Y, Wang H, Liang Q. Wang Q, et al. Front Immunol. 2018 Sep 27;9:2091. doi: 10.3389/fimmu.2018.02091. eCollection 2018. Front Immunol. 2018. PMID: 30319603 Free PMC article. - Hyperoside exerts anti-inflammatory and anti-arthritic effects in LPS-stimulated human fibroblast-like synoviocytes in vitro and in mice with collagen-induced arthritis.
Jin XN, Yan EZ, Wang HM, Sui HJ, Liu Z, Gao W, Jin Y. Jin XN, et al. Acta Pharmacol Sin. 2016 May;37(5):674-86. doi: 10.1038/aps.2016.7. Epub 2016 Apr 4. Acta Pharmacol Sin. 2016. PMID: 27041460 Free PMC article. - Taraxasterol suppresses inflammation in IL-1β-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice.
Chen J, Wu W, Zhang M, Chen C. Chen J, et al. Int Immunopharmacol. 2019 May;70:274-283. doi: 10.1016/j.intimp.2019.02.029. Epub 2019 Mar 6. Int Immunopharmacol. 2019. PMID: 30851708 - Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.
Joosten LA, Lubberts E, Helsen MM, Saxne T, Coenen-de Roo CJ, Heinegård D, van den Berg WB. Joosten LA, et al. Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article. - The suppressive effects of Saposhnikovia divaricata (Fangfeng) chromone extract on rheumatoid arthritis via inhibition of nuclear factor-κB and mitogen activated proteinkinases activation on collagen-induced arthritis model.
Kong X, Liu C, Zhang C, Zhao J, Wang J, Wan H, Zhu H, Zhang P, Chen W, Xiao Y, Lin N. Kong X, et al. J Ethnopharmacol. 2013 Jul 30;148(3):842-50. doi: 10.1016/j.jep.2013.05.023. Epub 2013 May 25. J Ethnopharmacol. 2013. PMID: 23711830
Cited by
- Dynamic synovial fibroblasts are modulated by NBCn1 as a potential target in rheumatoid arthritis.
Ji M, Ryu HJ, Baek HM, Shin DM, Hong JH. Ji M, et al. Exp Mol Med. 2022 Apr;54(4):503-517. doi: 10.1038/s12276-022-00756-6. Epub 2022 Apr 12. Exp Mol Med. 2022. PMID: 35414711 Free PMC article. - Augmented ERAD (ER-associated degradation) activity in chondrocytes is necessary for cartilage development and maintenance.
Sim HJ, Cho C, Kim HE, Hong JY, Song EK, Kwon KY, Jang DG, Kim SJ, Lee HS, Lee C, Kwon T, Yang S, Park TJ. Sim HJ, et al. Sci Adv. 2022 Jan 21;8(3):eabl4222. doi: 10.1126/sciadv.abl4222. Epub 2022 Jan 21. Sci Adv. 2022. PMID: 35061535 Free PMC article. - N-benzyl-N-methyldecan-1-amine and its derivative mitigate 2,4- dinitrobenzenesulfonic acid-induced colitis and collagen-induced rheumatoid arthritis.
Kim JE, Kang C, Budluang P, Yawut N, Cho IR, Choi YJ, Kim J, Ju S, Lee B, Sohn DH, Yim HS, Lee KW, Han J, Jung Y, Kang HY, Park JK, Jung Y, Hwang DY, Chung YH. Kim JE, et al. Front Pharmacol. 2023 Apr 20;14:1095955. doi: 10.3389/fphar.2023.1095955. eCollection 2023. Front Pharmacol. 2023. PMID: 37153778 Free PMC article. - ZhiJingSan Inhibits Osteoclastogenesis via Regulating RANKL/NF-κB Signaling Pathway and Ameliorates Bone Erosion in Collagen-Induced Mouse Arthritis.
Ling Y, Yang J, Hua D, Wang D, Zhao C, Weng L, Yue D, Cai X, Meng Q, Chen J, Sun X, Kong W, Zhu L, Cao P, Hu C. Ling Y, et al. Front Pharmacol. 2021 May 28;12:693777. doi: 10.3389/fphar.2021.693777. eCollection 2021. Front Pharmacol. 2021. PMID: 34122118 Free PMC article. - Potential biological functions and future perspectives of sialylated milk oligosaccharides.
Nguyen TLL, Nguyen DV, Heo KS. Nguyen TLL, et al. Arch Pharm Res. 2024 Apr;47(4):325-340. doi: 10.1007/s12272-024-01492-3. Epub 2024 Apr 1. Arch Pharm Res. 2024. PMID: 38561494 Review.
References
- Calabrese LH, Rose‐John S (2014). IL‐6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol 10: 720–727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical