IP3 Receptors Preferentially Associate with ER-Lysosome Contact Sites and Selectively Deliver Ca2+ to Lysosomes - PubMed (original) (raw)
IP3 Receptors Preferentially Associate with ER-Lysosome Contact Sites and Selectively Deliver Ca2+ to Lysosomes
Peace Atakpa et al. Cell Rep. 2018.
Abstract
Inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) allow extracellular stimuli to redistribute Ca2+ from the ER to cytosol or other organelles. We show, using small interfering RNA (siRNA) and vacuolar H+-ATPase (V-ATPase) inhibitors, that lysosomes sequester Ca2+ released by all IP3R subtypes, but not Ca2+ entering cells through store-operated Ca2+ entry (SOCE). A low-affinity Ca2+ sensor targeted to lysosomal membranes reports large, local increases in cytosolic [Ca2+] during IP3-evoked Ca2+ release, but not during SOCE. Most lysosomes associate with endoplasmic reticulum (ER) and dwell at regions populated by IP3R clusters, but IP3Rs do not assemble ER-lysosome contacts. Increasing lysosomal pH does not immediately prevent Ca2+ uptake, but it causes lysosomes to slowly redistribute and enlarge, reduces their association with IP3Rs, and disrupts Ca2+ exchange with ER. In a "piston-like" fashion, ER concentrates cytosolic Ca2+ and delivers it, through large-conductance IP3Rs, to a low-affinity lysosomal uptake system. The involvement of IP3Rs allows extracellular stimuli to regulate Ca2+ exchange between the ER and lysosomes.
Keywords: Ca(2+); IP(3) receptor; concanamycin A; endoplasmic reticulum; genetically encoded Ca(2+) sensor; lysosome; membrane contact site; proximity ligation assay; store-operated Ca(2+) entry.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
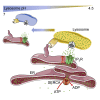
Graphical abstract
Figure 1
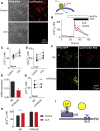
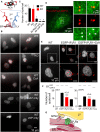
Inhibition of Lysosomal V-ATPase Potentiates Cytosolic Ca2+ Signals Evoked by IP3Rs (A) Bright-field and wide-field fluorescence images of HEK cells loaded with LysoTracker Red (100 nM, 10 min) with or without CcA (1 μM, 1 hr). Images are typical of three experiments. (B) Fluo 8-loaded HEK cells were treated with CcA (1 μM, 1 hr) in HBS before addition of 1,2-bis(o-aminophenoxy)ethane-_N,N,N′,N′_-tetraacetic acid (BAPTA) (2.5 mM) to chelate extracellular Ca2+ and then CCh (1 mM) to stimulate IP3 formation. Typical traces show mean ± SD from three wells in one experiment. (C and D) Summary results show effects of CcA on basal [Ca2+]c (C) and peak increase in [Ca2+]c (Δ[Ca2+]c) evoked by CCh (D). Results show paired individual values (each from three determinations) and the mean (n = 7, line). ∗p < 0.05, paired Student’s t test. (E) Expression of mRNA for ATP6V0C relative to GAPDH in cells treated with non-silencing siRNA (NS) or siRNA for ATP6V0C. Mean ± SEM, n = 6. ∗p < 0.05, paired Student’s t test. (F) TIRFM images show effects of siRNAs in HEK cells expressing TPC2-GFP or stained with LysoTracker Red (100 nM, 10 min). Images are typical of three experiments. (G) Effects of siRNA on basal [Ca2+]c (n = 6, each with three determinations). (H) Effects of siRNA on Δ[Ca2+]c evoked by CCh alone or after CcA (1 μM, 1 hr) (mean ± SEM, n = 5, each with three determinations). ∗p < 0.05, two-way ANOVA with Bonferroni test. (I) Results and Figure S1 demonstrate that lysosomes (LY) selectively sequester Ca2+ released from ER through IP3Rs, but not Ca2+ entering the cell through SOCE. See also Figure S1.
Figure 2
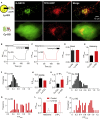
IP3Rs Selectively Deliver Ca2+ to Some Lysosomes (A) Wide-field fluorescence images of HeLa cells expressing TPC2-RFP with Ly-GG or Cy-GG. (B and C) Recordings from HeLa cells expressing Cy-GG (B) or Ly-GG (C) showing responses to histamine (100 μM) in Ca2+-free HBS and then ionomycin (10 μM) with 2 mM CaCl2 (to saturate the sensor). Results (F/Fmax, where Fmax is response after ionomycin) show responses of a single tracked lysosome (C) or a similarly sized cytosolic region of interest (ROI) (B) (see Video S1). (D and E) Summary results (mean ± SEM) show basal fluorescence (D) (3 experiments with 198 ROIs for Cy-GG and 83 tracks for Ly-GG) and peak fluorescence signals evoked by histamine (E) for Cy-GG (3 experiments with 198 ROIs) and Ly-GG (4 experiments with 83 lysosome tracks). ∗p < 0.05, Student’s t test. (F and G) Distribution of peak F/Fmax values for Cy-GG (F) and Ly-GG (G) in cells stimulated with histamine. Distribution of Ly-GG fluorescence values is significantly different from a normal distribution (Kolmogorov-Smirnov normality test, p = 0.0018), whereas Cy-GG fluorescence is consistent with a normal distribution (p > 0.1). (H) Peak responses from tracked regions for Cy-GG and Ly-GG for cells in which SOCE was evoked by restoration of extracellular Ca2+ (2 mM) to cells treated with thapsigargin (1 μM, 15 min) in Ca2+-free HBS. Mean ± SEM from at least three experiments (30 tracks for Ly-GG and 45 ROIs for Cy-GG). (I and J) Distribution of peak F/Fmax values for Cy-GG (I) and Ly-GG (J) after photolysis of ci-IP3 in Ca2+-free HBS (n = 59 ROIs from 4 dishes for Cy-GG and 49 tracks from 3 dishes for Ly-GG). (K) Summary results (mean ± SEM). ∗p < 0.05, Student’s t test. (L) Effects of U73122 (10 μM, 20 min) on peak Ly-GG signals evoked by histamine (100 μM) or photolysis of ci-IP3. Mean ± SEM, n = 3–4. ∗p < 0.05, Student’s t test, relative to control. See also Figure S1 and Video S1.
Figure 3
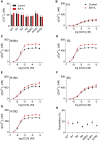
Lysosomes Sequester Ca2+ Released by All IP3R Subtypes (A) Basal [Ca2+]c in HEK cells expressing only the indicated IP3R subtypes and treated with bafilomycin A1 (Baf A1, 1 μM, 1 hr). Mean ± SEM. n = 3, each with three determinations. (B–G) Effects of Baf A1 (1 μM, 1 hr) on Ca2+ release evoked by CCh in HEK cells expressing only the indicated IP3R subtypes. Mean ± SEM, n = 6. The code in (B) applies also to (C)–(G). Similar results from WT cells are shown in Figure S1B. (H) Summary results show the potentiating effect of Baf A1 on the peak CCh-evoked Ca2+ signal. Results (mean ± SD) show the increase in amplitude of Ca2+ signal in the presence of Baf A1 as a percentage of the control response. WT, wild-type. See also Figure S1.
Figure 4
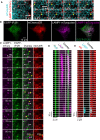
IP3Rs Associate with ER-Lysosome Contacts (A) TIRFM images of HeLa cell expressing mTurquoise-ER and LAMP1-mCherry showing that most lysosomes associate with ER and maintain contact as they move (yellow arrows). Images are shown at 8.4-s intervals (see Video S2). (B) TIRFM images of EGFP-IP3R1-HeLa cell expressing markers for lysosomes (mTurquoise-LAMP1) and ER (mCherry-ER). Merged image shows overlay of EGFP-IP3R1 and lysosomes. (C) Time-lapse TIRFM images (10-s intervals from Video S4) of boxed region in (B) show distribution of EGFP-IP3R1, lysosomes, and ER. Arrows show examples of mobile lysosomes (yellow) and those that remain immobile for sustained periods (white). Immobile lysosomes coincide with IP3R puncta. Mobile IP3R puncta are not visible in these images because of the long capture intervals (3.3 s). (D and E) Kymograms (3.3-s intervals) of boxed regions in (C) show a lysosome that is stationary for a prolonged period adjacent to an EGFP-IP3R1 punctum (D, from white box in C), whereas another lysosome pauses only briefly at adjacent ER (E, from yellow box in C). See also Figures S2, S3, and S6, and Videos S2, S3, S4, S5, S6, S7, and S8.
Figure 5
PLA Analyses Show IP3Rs at ER-Lysosome MCSs (A) PLA uses primary antibodies that recognize proteins in ER or lysosome (LY) membranes. Complementary oligonucleotides conjugated to secondary antibodies hybridize only if they are close to each other. Ligation of the hybridized strands then allows rolling circle amplification (RCA) of the oligonucleotide and incorporation of the red fluorescent probe. (B) Images of HEK cells with (WT) and without (KO) IP3Rs from PLA analyses of VAMP-associated protein A (VAP-A) proximity to Rab7. Confocal maximum intensity Z-projections show PLA spots (red) and nuclei (gray). Effects of CcA (1 μM, 1 hr) and omission of either primary antibody (Ab) are shown. (C) Summary results (mean ± SD, n = 15–25 cells from three experiments [two experiments for single-antibody controls]) show number of PLA spots/cell. ∗p < 0.05, one-way ANOVA with Dunnett’s test, relative to WT control. (D) Confocal section of PLA in EGFP-IP3R1 HeLa cells shows VAP-A proximity to LAMP1 (red) and endogenously tagged IP3R1 (green). Boxed areas are shown enlarged to illustrate the coincidence of EGFP-IP3R puncta with PLA spots (ER-lysosome MCS) (Manders’s split coefficient, 0.70 ± 0.21, n = 18 cells). (E) Confocal maximum intensity Z-projections of PLA in EGFP-IP3R1 HeLa cells using antibodies to GFP and LAMP1 to show their proximity (red spots). Nucleus is shown in gray. Effects of CcA (1 μM, 1 hr) and of performing the same PLA in wild-type (WT) HeLa cells without EGFP-IP3R1 are also shown. (F) Summary PLA results using Rab7 (left panel) or LAMP1 (right) to identify lysosomes and either GFP (from EGFP-IP3R1) or VAP-A to identify ER (shown by bars above the histograms). Mean ± SD, n = 25–97 cells from 3–5 experiments. ∗∗∗p < 0.001, Student’s t test for CcA-treated relative to matched control EGFP-IP3R1-HeLa cell. (G) Most lysosomes (LY) are closely associated, aided by tethers, with ER at MCS. Small clusters of IP3Rs associate with these MCS, but IP3Rs are not required for their assembly. WT, wild-type. See also Figures S2 and S6 and Videos S2, S3, S4, S5, S6, S7, and S8.
Figure 6
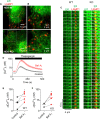
IP3Rs Are Not Required for ER-Lysosome Contacts (A) TIRFM images of HEK cells with (WT) and without IP3Rs (KO), expressing LAMP1-mCherry and EGFP-ER. (B) Enlargements of boxed region in (A) show associations of lysosomes and ER. (C) Time series (3-s intervals) of boxed regions in (B) show dynamics of ER-lysosome interactions (from Videos S9 and S10). (D) HEK cells were treated with bafilomycin A1 (Baf A1, 1 μM, 1 hr) in HBS before addition of BAPTA (2.5 mM) and then thapsigargin (1 μM). Mean ± SD from three wells in one experiment. (E and F) Summary results show peak thapsigargin-evoked Ca2+ release in WT (E) and KO cells (F) as paired values (each with three determinations) and mean (n = 4, line). ∗p < 0.05, paired Student’s t test. See also Figures S5 and S6 and Videos S9 and S10.
Figure 7
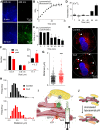
Increasing Lysosomal pH Slowly Redistributes Lysosomes and Attenuates Ca2+ Handling (A) Lysosomes of HEK cells were loaded with fluorescein-dextran (pKa = 6.4). Wide-field images show fluorescence recorded at pH-sensitive (λex = 488 nm) and -insensitive (λex = 425 nm) wavelengths before (0 min) and after CcA (1 μM, 1 hr). Images are typical of three experiments. (B) Summary results (mean ± SEM, n = 3) show time course of lysosomal pH changes after CcA as fluorescence ratios (R = F488/F425), which increase as pH increases. R0 is R determined before CcA. (C) Effects of CcA (1 μM) using LysoTracker red (50 nM, 1 hr) fluorescence, which declines as lysosomal pH increases. Mean ± SEM, n = 4–10. ∗p < 0.05, one-way ANOVA with Dunnett’s post hoc test, relative to t = 0. (D) Parallel analysis of CcA (1 μM) effects on peak increase in [Ca2+]c evoked by CCh (1 mM) in Ca2+-free HBS. Results (mean ± SEM, n = 4–9, with three determinations) show increase in peak Ca2+ signal in the presence of CcA relative to that in its absence, as percentage of response evoked by CCh alone. (E) Confocal z stack of HEK cells expressing LAMP1-mCherry before and after CcA (1 μM, 1 hr). Nuclei stained with NucBlue. The appearance of larger lysosomes in the cell periphery (white arrows) following CcA (1 μM, 1 hr) was clearly observed in four of six cells. (F) Effect of CcA (1 μM, 1 hr) on distribution of lysosome sizes (reported as Feret diameter, see STAR Methods). Results are from 721 (control) and 617 lysosomes (CcA-treated) from 4 cells in 4 independent experiments. Inset shows enlargement of the largest size category. ∗p < 0.05, Student’s t test. A similar analysis using lysosomes identified by an endocytosed fluorophore is shown in Figure S7. (G and H) Effects of CcA (1 μM, 1 hr) on the distance between each lysosome and the nearest IP3R punctum. Because these distances are reported as centroid-centroid separations, they can be smaller than the diffraction limit of the microscope. Results from four cells, with ∼20 × 20 μm analyzed in each, show each measurement and mean (line) (G) and frequency distributions (H). ∗∗∗p < 0.001, two-tailed Student’s t test. (I) Delivery of Ca2+ through IP3Rs or unidentified “leak channels” into MCSs provides a low-affinity Ca2+ uptake system in lysosomes with the high local [Ca2+] required for its activity. The ER, with its high-affinity Ca2+ pump (SERCA), accumulates Ca2+ from the cytosol and delivers it at high local concentration to the surface of lysosomes through large-conductance IP3Rs. ER behaves as an ATP-powered piston to concentrate Ca2+ around the lysosomal uptake system. (J) Dissipating lysosomal pH gradient does not immediately prevent lysosomal Ca2+ uptake, but slowly disrupts junctions within which it occurs. See also Figure S7.
Comment in
- Getting close. Lysosome-ER contact sites tailor Ca2+ signals.
Patel S. Patel S. Cell Calcium. 2019 Jun;80:194-196. doi: 10.1016/j.ceca.2019.02.003. Epub 2019 Feb 8. Cell Calcium. 2019. PMID: 30795844 No abstract available.
Similar articles
- IP3 receptors and store-operated Ca2+ entry: a license to fill.
Taylor CW, Machaca K. Taylor CW, et al. Curr Opin Cell Biol. 2019 Apr;57:1-7. doi: 10.1016/j.ceb.2018.10.001. Epub 2018 Oct 24. Curr Opin Cell Biol. 2019. PMID: 30368032 Review. - The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes.
Garrity AG, Wang W, Collier CM, Levey SA, Gao Q, Xu H. Garrity AG, et al. Elife. 2016 May 23;5:e15887. doi: 10.7554/eLife.15887. Elife. 2016. PMID: 27213518 Free PMC article. - IP3 receptors and Ca2+ entry.
Thillaiappan NB, Chakraborty P, Hasan G, Taylor CW. Thillaiappan NB, et al. Biochim Biophys Acta Mol Cell Res. 2019 Jul;1866(7):1092-1100. doi: 10.1016/j.bbamcr.2018.11.007. Epub 2018 Nov 15. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30448464 Review. - GPN does not release lysosomal Ca2+ but evokes Ca2+ release from the ER by increasing the cytosolic pH independently of cathepsin C.
Atakpa P, van Marrewijk LM, Apta-Smith M, Chakraborty S, Taylor CW. Atakpa P, et al. J Cell Sci. 2019 Feb 1;132(3):jcs223883. doi: 10.1242/jcs.223883. J Cell Sci. 2019. PMID: 30617110 Free PMC article. - Structure and Function of IP3 Receptors.
Prole DL, Taylor CW. Prole DL, et al. Cold Spring Harb Perspect Biol. 2019 Apr 1;11(4):a035063. doi: 10.1101/cshperspect.a035063. Cold Spring Harb Perspect Biol. 2019. PMID: 30745293 Free PMC article. Review.
Cited by
- Endoplasmic Reticulum Membrane and Contact Site Dynamics in Autophagy Regulation and Stress Response.
Morel E. Morel E. Front Cell Dev Biol. 2020 May 29;8:343. doi: 10.3389/fcell.2020.00343. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32548114 Free PMC article. Review. - Inter-organellar Communication in Parkinson's and Alzheimer's Disease: Looking Beyond Endoplasmic Reticulum-Mitochondria Contact Sites.
Vrijsen S, Vrancx C, Del Vecchio M, Swinnen JV, Agostinis P, Winderickx J, Vangheluwe P, Annaert W. Vrijsen S, et al. Front Neurosci. 2022 Jun 21;16:900338. doi: 10.3389/fnins.2022.900338. eCollection 2022. Front Neurosci. 2022. PMID: 35801175 Free PMC article. - Intracellular Ca2+ signalling: unexpected new roles for the usual suspect.
Moccia F, Fiorio Pla A, Lim D, Lodola F, Gerbino A. Moccia F, et al. Front Physiol. 2023 Jul 27;14:1210085. doi: 10.3389/fphys.2023.1210085. eCollection 2023. Front Physiol. 2023. PMID: 37576340 Free PMC article. Review. - STAT3 Localizes in Mitochondria-Associated ER Membranes Instead of in Mitochondria.
Su Y, Huang X, Huang Z, Huang T, Xu Y, Yi C. Su Y, et al. Front Cell Dev Biol. 2020 Apr 22;8:274. doi: 10.3389/fcell.2020.00274. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32391361 Free PMC article. - TMBIM6 (transmembrane BAX inhibitor motif containing 6) enhances autophagy through regulation of lysosomal calcium.
Kim HK, Lee GH, Bhattarai KR, Lee MS, Back SH, Kim HR, Chae HJ. Kim HK, et al. Autophagy. 2021 Mar;17(3):761-778. doi: 10.1080/15548627.2020.1732161. Epub 2020 Mar 13. Autophagy. 2021. PMID: 32167007 Free PMC article.
References
- Alpy F., Rousseau A., Schwab Y., Legueux F., Stoll I., Wendling C., Spiegelhalter C., Kessler P., Mathelin C., Rio M.C. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J. Cell Sci. 2013;126:5500–5512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous