Exploration of CTCF post-translation modifications uncovers Serine-224 phosphorylation by PLK1 at pericentric regions during the G2/M transition - PubMed (original) (raw)
Exploration of CTCF post-translation modifications uncovers Serine-224 phosphorylation by PLK1 at pericentric regions during the G2/M transition
Brian C Del Rosario et al. Elife. 2019.
Abstract
The zinc finger CCCTC-binding protein (CTCF) carries out many functions in the cell. Although previous studies sought to explain CTCF multivalency based on sequence composition of binding sites, few examined how CTCF post-translational modification (PTM) could contribute to function. Here, we performed CTCF mass spectrometry, identified a novel phosphorylation site at Serine 224 (Ser224-P), and demonstrate that phosphorylation is carried out by Polo-like kinase 1 (PLK1). CTCF Ser224-P is chromatin-associated, mapping to at least a subset of known CTCF sites. CTCF Ser224-P accumulates during the G2/M transition of the cell cycle and is enriched at pericentric regions. The phospho-obviation mutant, S224A, appeared normal. However, the phospho-mimic mutant, S224E, is detrimental to mouse embryonic stem cell colonies. While ploidy and chromatin architecture appear unaffected, S224E mutants differentially express hundreds of genes, including p53 and p21. We have thus identified a new CTCF PTM and provided evidence of biological function.
Keywords: CTCF; PLK1; cell cycle; chromosome architecture; chromosomes; gene expression; mouse; post-translational modification.
© 2019, Del Rosario et al.
Conflict of interest statement
BD, AK, AD, AA, FW, RS No competing interests declared, CF Employee of Cell Signaling Technologies. There are no other competing interests to declare. JL Reviewing editor, eLife, and is a cofounder and member of the Scientific Advisory Boards of Translate Bio and Fulcrum Therapeutics.
Figures
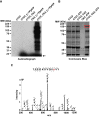
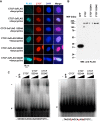
Figure 1.. Murine CTCF is phosphorylated at Ser224.
(A) GFP microscopy of co-inducible CTCF-3xFLAG, EGFP MEFs ± 1 μg/mL doxycycline for 48 hr. Bar, 25 μm. (B) FLAG and CTCF immunofluorescence accompanying (A). Bar, 10 μm. (C) FLAG, CTCF, and β-actin western blot of whole cell extracts from inducible CTCF-3XFLAG MEFs treated up to 9 days with 1 μg/mL doxycycline. (D) Coomassie stained gel of anti-FLAG immunoprecipitate from CTCF-3xFLAG MEFs treated 72hrs ± 1μg/mL doxycycline. Red arrow, band analyzed by mass spectrometry. (E) Manually validated mass spectra of CTCF peptide Tyr214-Lys244 with y and b ions identified. Phosphorylation event at Ser224 indicated by red s. (F) ClustalX alignment of CTCF sequences from the indicated species. Shown is a 25 amino acid window centered on mouse Ser224 (red arrow).
Figure 1—figure supplement 1.. Casein kinase 2 (CK2) can phosphorylate CTCF Ser224.
(A) In vitro kinase assay autoradiograph of recombinant FLAG-CTCF-6xHis phosphorylated by CK2 with [γ-32P]ATP. Black arrow, free [γ-32P]ATP. (B) Coomassie Blue stained gel of FLAG-CTCF-6xHis phosphorylated with CK2 and cold ATP performed in parallel as in A. Red box, Full-length FLAG-CTCF-6xHis band excised for mass spectrometry analysis. (C) Manually validated mass spectra of CTCF peptide Thr215-Tyr226 with y and b ions identified. Phosphorylation at Ser224 indicated by red s.
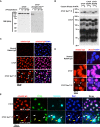
Figure 2.. CTCF Ser224-P is conserved and accumulates at G2/M.
(A) CTCF and CTCF Ser224-P western blots of MEF lysates treated with Lambda Protein Phosphatase for the indicated times. (B) CTCF and CTCF Ser224-P western blots of recombinant CTCF, CTCF S224A, and CTCF S224E ± Casein Kinase II in vitro phosphorylation. (C) Immunofluorescence performed on asynchronous MEFs with the indicated antibodies. Nuclei counterstained with DAPI. Bar, 50 μm. White arrows, prominent CTCF Ser224-P cells. (D) Immunofluorescence performed on asynchronous HEK293 with the indicated antibodies. Nuclei counterstained with DAPI. Bar, 20 μm. White arrows, prominent CTCF Ser224-P cells. (E) Immunofluorescence performed on asynchronous MEFs with the indicated antibodies. Nuclei counterstained with DAPI. Bar, 50 μm. % of n cells labeled with CTCF or CTCF Ser224-P antibodies indicated. White arrows, early G2. Yellow arrows, late G2.
Figure 2—figure supplement 1.. CTCF Ser224-P accumulates in G2 and has a subnuclear distribution comparable to total CTCF.
(A–D), Immunofluorescence performed on immortalized MEFs using the indicated antibodies. Nuclei counterstained with DAPI. Bar, 10 μm. (E) Immunofluorescence performed on asynchronous MEFs with the indicated antibodies. Nuclei counterstained with DAPI. Bar, 50 μm. White arrows (PCNA negative, speckled H3S10ph), early G2. Yellow arrows (PCNA negative, pronounced H3S10ph), late G2. Orange arrows (Speckled PCNA, no H3S10ph), S. Magenta arrows (Speckled PCNA, faint speckled H3S10ph), late S/early G2. (F) Immunofluorescence performed on TST-1 mESCs with the indicated antibodies. Cells were treated 20 hr with 10 μM RO-3306 or DMSO alone control. Bar, 25 μm.
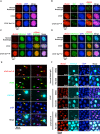
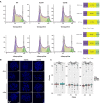
Figure 3.. CTCF Ser224 is phosphorylated by PLK1 and prominently labels pericentric chromatin.
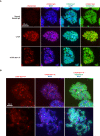
(A) Graphic comparison of PLK1 consensus substrate sequence with CTCF D220-F228. Green S/T with yellow encircled P, phosphorylation site at position 0. Red D/E, aspartic or glutamic acid. Blue Φ, hydrophobic amino acid. (B) Amino acid sequence identity heat map for the conserved kinases PLK1, ATM, and GSK3B. Nephrozoa species aligned in Figure 1F shown. (C) CTCF, CTCF Ser224-P, PCNA, and H3S10ph immunofluorescence performed on MEFs treated with DMSO or BI 6727 at the indicated concentrations for 12 hr. Nuclei counterstained with DAPI. Bar, 20 μm. % of n cells labeled with CTCF, CTCF Ser224-P, PCNA, or H3S10ph antibodies indicated. (D) PLK1 in vitro kinase assay with CTCF or dephosphorylated Casein substrates. Red *, phosphorylated CTCF. Red **, autophosphorylated PLK1. Red ***, phosphorylated casein. (E) In vitro kinase assay performed in parallel to (D without radioactive isotope. SDS-PAGE gel Coomassie stained. Red box, CTCF band excised for mass spectrometry analysis. (F) Manually validated mass spectra of CTCF peptide Tyr214-Lys244 with y and b ions identified. Phosphorylation event at Ser224 indicated by red s. (G) Immunofluorescence performed on TST-1 mESC metaphase chromosomes with the indicated antibodies. DNA stained with DAPI. (H) CTCF Ser224-P and H3K27me3 co-stain from (G) deconvolved.
Figure 3—figure supplement 1.. CTCF Ser224-P is enriched at pericentric regions of metaphase chromosomes.
(A) Immunofluorescence performed on TST-1 mESC metaphase chromosomes using the indicated antibodies. DNA stained with DAPI. CTCF Ser224-P images are replicated from Figure 3G and are shown for comparison purposes. Bar, 20 μm. (B) Two additional examples of immunofluorescence on TST-1 mESC metaphase chromosomes using anti-CTCF Ser224-P and anti-H3K27me3 antibodies. DNA stained with DAPI. Bar, 10 μm.
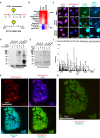
Figure 4.. CTCF Ser224-P occupies a fraction of CTCF sites outside of pericentric chromatin in interphase.
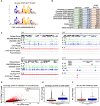
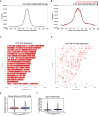
(A) De novo CTCF Ser224-P motif logo determined with MEME-ChIP (top). JASPAR indexed CTCF motif logo (bottom). (B) % distribution of CTCF (blue) and CTCF Ser224-P (orange) ChIP-seq peaks across genomic features. % distribution of these features in the genome is shown for comparison (green). (C) Four example screenshots showing CTCF (blue) and CTCF Ser224-P (red) ChIP-seq coverage tracks and called peaks. Intersected CTCF and CTCF Ser224-P peaks (purple) are also shown. ENCODE CTCF ChIP-seq coverage (green), Refseq Genes (black) and ENCODE RNA-seq (gray) are shown for reference. Chromosome number and window scale are indicated. (D) CTCF versus CTCF Ser224-P ChIP-seq coverage on CTCF (black) and shared CTCF and CTCF Ser224-P ChIP-seq peaks (red). R-squared values for both sets of peaks are shown. (E) CTCF ChIP-seq coverage on CTCF versus shared CTCF and CTCF Ser224-P ChIP-seq peaks (p<2.2×10−16, Wilcoxon rank sum test). (F) Number of CTCF motifs found in CTCF versus shared CTCF and CTCF Ser224-P ChIP-seq peaks (p<2.2×10−16, Wilcoxon rank sum test).
Figure 4—figure supplement 1.. CTCF Ser224-P occupies known CTCF binding sites.
(A) CentriMo site probability distribution plot generated from CTCF ChIP-seq called peaks (z = 6) with the observed consensus CTCF motif plotted (black trace). (B) Site probability distribution plot generated from CTCF Ser224-P ChIP-seq called peaks (z = 6) with both de novo identified (red) and consensus CTCF (black) motifs plotted. (C–D) CTCF and CTCF Ser224-P ChIP-seq peak (z = 6) distribution plots across all chromosomes. (E) Average conservation of CTCF motifs falling in CTCF versus CTCF Ser224-P ChIP-seq peaks (p-value>0.05, Wilcoxon rank sum test). (F) Length of CTCF peaks versus shared CTCF and CTCF Ser224-P peaks (p-value<2.2×10−16, Wilcoxon rank sum test).
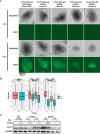
Figure 5.. CTCF S224E phosphomimic mutation is poorly tolerated by dividing cells.
(A) Representative brightfield and EGFP images of F1-2.1 mESCs carrying a dox-inducible wild type CTCF, S224A or S224E transgene grown for six days with (bottom) or without (top) doxycycline. Two independent S224A and S224E clones are shown. (B) Quantification of colony diameters in microns of cell lines shown in (A), grown for six days with (blue) or without (red) doxycycline. Student’s t-test was used to calculate p-values between indicated samples, with not significant (N.S.) p-values being >0.05. (C) Western blot measuring FLAG and CTCF protein levels of cell lines shown in (A). GAPDH is shown as a loading control.
Figure 6.. CTCF Ser224 is nonessential to nuclear import and DNA binding.
(A) FLAG and CTCF immunofluorescence performed on rtTA MEFs with inducible CTCF-3xFLAG transgenes (wild type, S224A, or S224E). Nuclei counterstained with DAPI. Bar, 10 μm. (B) FLAG western blot of recombinant FLAG-tagged GFP and CTCF (wild type, S224A, or S224E). (C) DNA EMSA using RS14C (left) and RS14C mutant (right) probes. Red lowercase letters, mutated positions. 2 pmole GFP and 0.5, 1, or 2 pmole CTCF (wild type, S224A, or S224E) were used. *, CTCF shifted probe. #, free probe.
Figure 7.. Overexpression of CTCF, S224A or S224E does not impact cell cycle progression or ploidy.
(A) Cell cycle profiles of F1-2.1 mESCs carrying a dox inducible CTCF, S224A or S224E transgene grown for six days with (bottom) or without (top) dox induction. Quantification of percent of cells in each stage of the cell cycle indicated in bar graph to the right. (B) Representative metaphase spreads of cells profiled in A grown for six days with (bottom) or without (top) dox induction. (C) Quantification of chromosome counts for cells profiled in A grown for six days with (bottom) or without (top) dox induction. Wilcoxon rank sum test was used to calculate p-values between indicated samples, with not significant (N.S.) p-values being >0.05.
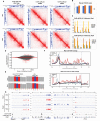
Figure 8.. Impact of overexpression of CTCF, S224A and S224E on three-dimensional chromatin structure and gene expression.
(A) Hi-C2 interaction maps at 25 kb resolution of the Mecp2 TAD in F1-2.1 mESCs carrying dox-inducible wild type, S224A or S224E CTCF-3xFLAG transgenes grown for 2 days with (bottom) and without (top) doxycycline. CTCF and CTCF Ser224-P ChIP-seq tracks are shown for comparison. Black arrows indicate the left border of a sub-TAD domain bound at both borders by CTCF and at one border by CTCF Ser224-P. In addition, dotted lines and text in the WT -dox Hi-C2 interaction map indicate locations of ChIP-qPCR primers used in (C), with the Irak1 and Ikbkg lines also indicating the borders of the Mecp2 TAD scored in (B). (B) TAD scores for the Mecp2 TAD for the Hi-C2 interaction maps in A, with higher TAD score indicating a stronger TAD. (C) CTCF and FLAG ChIP-qPCR (% Pulldown) in F1-2.1 mESCs carrying dox-inducible S224E CTCF-3xFLAG grown for 2 days with or without doxycycline. Cirbp and Ccnd indicate positive control regions for CTCF binding, while Oct4 indicates a negative control region. Flna, Irak1, and Ikbkg regions are as indicated in (A). (D) MA plot of RNA-seq expression changes in F1-2.1 mESCs carrying dox-inducible CTCF S224E transgene after 6 days of dox induction. Points in red are DE genes (adjusted p-value<0.01). (E) Coverage of RNA-seq reads over codon 224 of CTCF with (left) and without (right) 6 days of dox induction. The number of reads with A (green), C (blue), G (gold), T (red) or N (grey) at positions 1, 2, and 3 of codon 224 are shown. (F) Metagene coverage of CTCF ChIP-seq reads (top) and CTCF Ser224-P ChIP-seq reads (bottom) over upregulated (red), downregulated (green), non differentially expressed (purple) and all (black) genes. (G) RNA-seq, CTCF and CTCF Ser224-P ChIP-seq coverage over two representative upregulated (left) and downregulated (right) genes.
Similar articles
- Suppression of Vimentin Phosphorylation by the Avian Reovirus p17 through Inhibition of CDK1 and Plk1 Impacting the G2/M Phase of the Cell Cycle.
Chiu HC, Huang WR, Liao TL, Wu HY, Munir M, Shih WL, Liu HJ. Chiu HC, et al. PLoS One. 2016 Sep 7;11(9):e0162356. doi: 10.1371/journal.pone.0162356. eCollection 2016. PLoS One. 2016. PMID: 27603133 Free PMC article. - M phase-specific phosphorylation of BRCA2 by Polo-like kinase 1 correlates with the dissociation of the BRCA2-P/CAF complex.
Lin HR, Ting NS, Qin J, Lee WH. Lin HR, et al. J Biol Chem. 2003 Sep 19;278(38):35979-87. doi: 10.1074/jbc.M210659200. Epub 2003 Jun 17. J Biol Chem. 2003. PMID: 12815053 - Polo-like kinase 1 regulates mitotic arrest after UV irradiation through dephosphorylation of p53 and inducing p53 degradation.
Chen J, Dai G, Wang YQ, Wang S, Pan FY, Xue B, Zhao DH, Li CJ. Chen J, et al. FEBS Lett. 2006 Jun 26;580(15):3624-30. doi: 10.1016/j.febslet.2006.05.047. Epub 2006 May 30. FEBS Lett. 2006. PMID: 16753148 - The substrates of Plk1, beyond the functions in mitosis.
Liu XS, Song B, Liu X. Liu XS, et al. Protein Cell. 2010 Nov;1(11):999-1010. doi: 10.1007/s13238-010-0131-x. Epub 2010 Dec 10. Protein Cell. 2010. PMID: 21153517 Free PMC article. Review. - Aurora-A and hBora join the game of Polo.
Macurek L, Lindqvist A, Medema RH. Macurek L, et al. Cancer Res. 2009 Jun 1;69(11):4555-8. doi: 10.1158/0008-5472.CAN-09-0142. Cancer Res. 2009. PMID: 19487276 Review.
Cited by
- Balancing cohesin eviction and retention prevents aberrant chromosomal interactions, Polycomb-mediated repression, and X-inactivation.
Kriz AJ, Colognori D, Sunwoo H, Nabet B, Lee JT. Kriz AJ, et al. Mol Cell. 2021 May 6;81(9):1970-1987.e9. doi: 10.1016/j.molcel.2021.02.031. Epub 2021 Mar 15. Mol Cell. 2021. PMID: 33725485 Free PMC article. - Function and regulation of transcription factors during mitosis-to-G1 transition.
Soares MAF, Oliveira RA, Castro DS. Soares MAF, et al. Open Biol. 2022 Jun;12(6):220062. doi: 10.1098/rsob.220062. Epub 2022 Jun 1. Open Biol. 2022. PMID: 35642493 Free PMC article. Review. - Molecular basis of CTCF binding polarity in genome folding.
Nora EP, Caccianini L, Fudenberg G, So K, Kameswaran V, Nagle A, Uebersohn A, Hajj B, Saux AL, Coulon A, Mirny LA, Pollard KS, Dahan M, Bruneau BG. Nora EP, et al. Nat Commun. 2020 Nov 5;11(1):5612. doi: 10.1038/s41467-020-19283-x. Nat Commun. 2020. PMID: 33154377 Free PMC article. - Jpx RNA regulates CTCF anchor site selection and formation of chromosome loops.
Oh HJ, Aguilar R, Kesner B, Lee HG, Kriz AJ, Chu HP, Lee JT. Oh HJ, et al. Cell. 2021 Dec 9;184(25):6157-6173.e24. doi: 10.1016/j.cell.2021.11.012. Epub 2021 Dec 1. Cell. 2021. PMID: 34856126 Free PMC article. - The PTM profiling of CTCF reveals the regulation of 3D chromatin structure by O-GlcNAcylation.
Tang X, Zeng P, Liu K, Qing L, Sun Y, Liu X, Lu L, Wei C, Wang J, Jiang S, Sun J, Chang W, Yu H, Chen H, Zhou J, Xu C, Fan L, Miao YL, Ding J. Tang X, et al. Nat Commun. 2024 Apr 1;15(1):2813. doi: 10.1038/s41467-024-47048-3. Nat Commun. 2024. PMID: 38561336 Free PMC article.
References
- Burke LJ, Zhang R, Bartkuhn M, Tiwari VK, Tavoosidana G, Kurukuti S, Weth C, Leers J, Galjart N, Ohlsson R, Renkawitz R. CTCF binding and higher order chromatin structure of the H19 locus are maintained in Mitotic chromatin. The EMBO Journal. 2005;24:3291–3300. doi: 10.1038/sj.emboj.7600793. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37-GM58839/NH/NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- NSF GRFP/National Science Foundation/International
- Herchel Smith Graduate Fellowship/Herchel Smith Endowment fund/International
- R01 GM058839/GM/NIGMS NIH HHS/United States
- R37 GM058839/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous