Growth factor stimulation promotes multivesicular endosome biogenesis by prolonging recruitment of the late-acting ESCRT machinery - PubMed (original) (raw)
Growth factor stimulation promotes multivesicular endosome biogenesis by prolonging recruitment of the late-acting ESCRT machinery
Kyle B Quinney et al. Proc Natl Acad Sci U S A. 2019.
Abstract
The formation of multivesicular endosomes (MVEs) mediates the turnover of numerous integral membrane proteins and has been implicated in the down-regulation of growth factor signaling, thereby exhibiting properties of a tumor suppressor. The endosomal sorting complex required for transport (ESCRT) machinery plays a key role in MVE biogenesis, enabling cargo selection and intralumenal vesicle (ILV) budding. However, the spatiotemporal pattern of endogenous ESCRT complex assembly and disassembly in mammalian cells remains poorly defined. By combining CRISPR/Cas9-mediated genome editing and live cell imaging using lattice light sheet microscopy (LLSM), we determined the native dynamics of both early- and late-acting ESCRT components at MVEs under multiple growth conditions. Specifically, our data indicate that ESCRT-0 accumulates quickly on endosomes, typically in less than 30 seconds, and its levels oscillate in a manner dependent on the downstream recruitment of ESCRT-I. Similarly, levels of the ESCRT-I complex also fluctuate on endosomes, but its average residency time is more than fivefold shorter compared with ESCRT-0. Vps4 accumulation is the most transient, however, suggesting that the completion of ILV formation occurs rapidly. Upon addition of epidermal growth factor (EGF), both ESCRT-I and Vps4 are retained at endosomes for dramatically extended periods of time, while ESCRT-0 dynamics are only modestly affected. Our findings are consistent with a model in which growth factor stimulation stabilizes late-acting components of the ESCRT machinery at endosomes to accelerate the rate of ILV biogenesis and attenuate signal transduction initiated by receptor activation.
Keywords: CRISPR/Cas9; ESCRT microdomain; epidermal growth factor receptor; lattice light sheet microscopy; organelle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
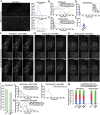
Generation of functionally tagged ESCRT subunits using CRISPR/Cas9-mediated genome editing. (A) Cartoon highlighting the placement of the HaloTag on three ESCRT subunits to track ESCRT-0 (green), ESCRT-I (blue), and Vps4 (purple) dynamics. The sequence of the flexible linker used in each case, which contains the tobacco etch virus protease recognition sequence, is also shown. (B) Representative immunoblot analyses of control and CRISPR/Cas9-modified cell lines (n = 3 each) using antibodies directed against Hrs (Left), Tsg101 (Center), Vps4B (Right), and β-actin (load control). (C) Control and CRISPR/Cas9-modified clonal cell lines were incubated in the presence or absence of 30 ng/mL EGF for 3 h following serum starvation, and extracts were immunoblotted using antibodies directed against EGFR (Top) and β-actin (Bottom, load control). (D) Quantification of the percentage of EGFR remaining after 3 h of EGF treatment in control and CRISPR/Cas9-modified clonal cell lines. Error bars represent mean ± SEM (n = 4 each). No statistically significant difference was found, as calculated using an ANOVA test. (E) Representative thin-section EM images of MVEs from control and CRISPR/Cas9-modified clonal cell lines (more than 35 MVEs and 200 ILVs examined in each). (Scale bar: 200 nm.) The size distribution of MVEs (F) and ILVs (G) in control and CRISPR/Cas9-modified clonal cell lines is shown. No statistically significant differences were found, as calculated using an ANOVA test.
Fig. 2.
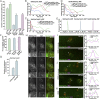
ESCRT-0 associates more stably with endosomes compared with downstream ESCRT complexes under normal growth conditions. (A) Representative immunoblot analysis (n = 3) of control and CRISPR/Cas9-modified cell lines using antibodies directed against HaloTag (Top) and β-actin (Bottom, load control). (B and C) Representative CRISPR/Cas9-modified cells imaged live using SFC optics following dye labeling with the JF549-HaloTag ligand (more than 15 different cells each, more than three biological replicates each). Maximum intensity projections of z-stacks (B) or individual confocal sections over time (C) are shown. Arrows highlight transient Vps4-positive endosomes. (Scale bars: 10 μm.) (D_–_F) Representative CRISPR/Cas9-modified cells imaged live using LLSM following dye labeling using the JF646-HaloTag ligand (more than 10 cells each, more than three biological replicates each). Projected z-stacks are shown for each time point. Arrows highlight the appearance of ESCRT-positive endosomes, and red circles denote their disappearance. (Scale bars: 5 μm; Insets, 2 μm.) (G) Quantification of the average duration of each HaloTag-ESCRT fusion protein on endosomes. Error bars represent mean ± SEM (more than 600 endosomes analyzed for each cell line).
Fig. 3.
ESCRT subunit dynamics are regulated by the action of downstream ESCRT complexes. (A, Left) Representative HaloTag-Hrs–expressing cells imaged live using STED microscopy following dye labeling using the SiR-HaloTag ligand (more than 10 cells imaged, more than three biological replicates). Arrows highlight endosomes of two different size classes (blue, less than 25th percentile; red, 25th–75th percentile). (Scale bar: 1 μm.) (A, Right) Fluorescence intensity based on line-scan analysis around the circumference of each is shown, reflecting the distribution of Hrs. (B_–_D) Averaged fluorescence intensity profiles of labeled endosomes classified by compartment size (more than 95% of Vps4B-HaloTag–labeled compartments were diffraction-limited). Only compartments that acquire and lose fluorescence during the imaging series were used (more than 350 endosomes per condition in more than 10 cells each, more than three biological replicates). (E) Individual residency times of Vps4B-HaloTag at endosomes are plotted (more than 140 endosomes, at least 10 different cells in more than three biological replicates). A black line indicates the average. (F_–_H) Representative CRISPR/Cas9-modified cells imaged live using LLSM following dye labeling with the JF646-HaloTag ligand and treatment with siRNA targeting either Tsg101 or both Vps4 isoforms (more than 10 cells each, more than three biological replicates each). Projected z-stacks are shown for each time point. Arrows highlight the appearance of ESCRT-positive endosomes, and red circles denote their disappearance. (Scale bars: 5 μm; Insets, 2 μm.) (I) Quantification of the average duration of HaloTag-Hrs on endosomes in control and Tsg101-depleted cells. Error bars represent mean ± SEM (more than 400 endosomes analyzed for each cell line, more than 10 cells imaged per condition in more than three biological replicates). **P < 0.01, as calculated using a Student’s t test. (J_–_L) Averaged fluorescence intensity profiles of labeled endosomes classified by compartment size under the conditions shown (relative to sizes determined under normal conditions). Only compartments that acquire and lose fluorescence during the imaging series were used (more than 400 endosomes per condition, more than 10 cells imaged per condition in more than three biological replicates). (M) Size distributions of ESCRT-positive endosomes under the conditions indicated (more 400 endosomes per condition, more than 10 different cells each in more than three biological replicates).
Fig. 4.
Growth factor stimulation alters ESCRT dynamics at endosomes. (A) Quantification of the average duration of each HaloTag-ESCRT fusion protein on endosomes in the presence or absence of EGF stimulation. Error bars represent mean ± SEM (more than 600 endosomes analyzed for each cell line, more than 10 cells each in more than three biological replicates). ***P < 0.005, as calculated using an ANOVA test. (B_–_D) Averaged fluorescence intensity profiles of labeled endosomes classified by compartment size following treatment with 30 ng/mL EGF. Only compartments that acquire and lose fluorescence during the imaging series were used (more than 350 endosomes per condition, more than 10 cells each in more than three biological replicates). (E) Time differences between Alexa Fluor 555-EGF and JF646-labeled HaloTag-ESCRT achieving maximum fluorescence intensity at endosomes are plotted (more than 400 endosomes analyzed for each cell line, more than 10 cells each in more than three biological replicates). (F) Percentages of ESCRT-labeled endosomes that exhibited EGF accumulation at any point during time-lapse imaging were calculated for each Halo-Tag ESCRT fusion. Error bars represent mean ± SEM (endosomes from more than 10 cells each in more than three biological replicates). (G) Quantification of the average duration of HaloTag-Tsg101 on endosomes, either EGF-positive or EGF-negative, following EGF stimulation. Error bars represent mean ± SEM (more than 500 endosomes analyzed, more than 10 cells each in more than three biological replicates). ***P < 0.005, as calculated using a t test. (H) Representative HaloTag-Hrs–expressing cells imaged live using LLSM following dye labeling using the JF646-HaloTag ligand and incubation with Alexa Fluor 555-EGF for 2 min, followed by washout (more 10 cells each, more than three biological replicates each). Projected z-stacks are shown for each time point. Arrows highlight HaloTag-Hrs–positive endosomes that also show accumulation of EGF. (Scale bar, 5 μm; Insets, 2 μm.) Representative HaloTag-Hrs–expressing cells (I and J) or HaloTag-Tsg101–expressing cells (K) imaged live using STED microscopy following dye labeling using the SiR-HaloTag ligand and incubation with Alexa Fluor 594-EGF (Left; more than 10 cells in more than three biological replicates) are shown. Fluorescence intensity based on line-scan analysis around the circumference of representative endosomes (Right, indicated by arrows on Left) is also shown, reflecting the relative distributions of each ESCRT complex and EGF. (Scale bars: I and J, 500 nm; K, 1 μm.)
Similar articles
- Ist1 regulates ESCRT-III assembly and function during multivesicular endosome biogenesis in Caenorhabditis elegans embryos.
Frankel EB, Shankar R, Moresco JJ, Yates JR 3rd, Volkmann N, Audhya A. Frankel EB, et al. Nat Commun. 2017 Nov 13;8(1):1439. doi: 10.1038/s41467-017-01636-8. Nat Commun. 2017. PMID: 29129923 Free PMC article. - Bro1 stimulates Vps4 to promote intralumenal vesicle formation during multivesicular body biogenesis.
Tseng CC, Dean S, Davies BA, Azmi IF, Pashkova N, Payne JA, Staffenhagen J, West M, Piper RC, Odorizzi G, Katzmann DJ. Tseng CC, et al. J Cell Biol. 2021 Aug 2;220(8):e202102070. doi: 10.1083/jcb.202102070. Epub 2021 Jun 23. J Cell Biol. 2021. PMID: 34160559 Free PMC article. - ESCRT-dependent cargo sorting at multivesicular endosomes.
Frankel EB, Audhya A. Frankel EB, et al. Semin Cell Dev Biol. 2018 Feb;74:4-10. doi: 10.1016/j.semcdb.2017.08.020. Epub 2017 Aug 8. Semin Cell Dev Biol. 2018. PMID: 28797838 Free PMC article. Review. - Concerted ESCRT and clathrin recruitment waves define the timing and morphology of intraluminal vesicle formation.
Wenzel EM, Schultz SW, Schink KO, Pedersen NM, Nähse V, Carlson A, Brech A, Stenmark H, Raiborg C. Wenzel EM, et al. Nat Commun. 2018 Jul 26;9(1):2932. doi: 10.1038/s41467-018-05345-8. Nat Commun. 2018. PMID: 30050131 Free PMC article. - The regulation of Endosomal Sorting Complex Required for Transport and accessory proteins in multivesicular body sorting and enveloped viral budding - An overview.
Ahmed I, Akram Z, Iqbal HMN, Munn AL. Ahmed I, et al. Int J Biol Macromol. 2019 Apr 15;127:1-11. doi: 10.1016/j.ijbiomac.2019.01.015. Epub 2019 Jan 4. Int J Biol Macromol. 2019. PMID: 30615963 Review.
Cited by
- Exosome-Derived LncRNAs in Lung Cancer.
Fan T, Sun N, He J. Fan T, et al. Front Oncol. 2020 Sep 23;10:1728. doi: 10.3389/fonc.2020.01728. eCollection 2020. Front Oncol. 2020. PMID: 33072553 Free PMC article. Review. - Extracellular Vesicles for Therapeutic Nucleic Acid Delivery: Loading Strategies and Challenges.
Oshchepkova A, Zenkova M, Vlassov V. Oshchepkova A, et al. Int J Mol Sci. 2023 Apr 14;24(8):7287. doi: 10.3390/ijms24087287. Int J Mol Sci. 2023. PMID: 37108446 Free PMC article. Review. - Nutrient deprivation alters the rate of COPII subunit recruitment at ER subdomains to tune secretory protein transport.
Kasberg W, Luong P, Swift KA, Audhya A. Kasberg W, et al. Nat Commun. 2023 Dec 8;14(1):8140. doi: 10.1038/s41467-023-44002-7. Nat Commun. 2023. PMID: 38066006 Free PMC article. - Exosome-mediated macrophage regulation for inflammatory bowel disease repair: a potential target of gut inflammation.
Ma F, Zhang S, Akanyibah FA, Zhang W, Chen K, Ocansey DKW, Lyu C, Mao F. Ma F, et al. Am J Transl Res. 2023 Dec 15;15(12):6970-6987. eCollection 2023. Am J Transl Res. 2023. PMID: 38186999 Free PMC article. Review. - Protein crowding mediates membrane remodeling in upstream ESCRT-induced formation of intraluminal vesicles.
Liese S, Wenzel EM, Kjos I, Rojas Molina R, Schultz SW, Brech A, Stenmark H, Raiborg C, Carlson A. Liese S, et al. Proc Natl Acad Sci U S A. 2020 Nov 17;117(46):28614-28624. doi: 10.1073/pnas.2014228117. Epub 2020 Nov 2. Proc Natl Acad Sci U S A. 2020. PMID: 33139578 Free PMC article.
References
- Bache KG, Raiborg C, Mehlum A, Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem. 2003;278:12513–12521. - PubMed
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. - PubMed
- Slagsvold T, et al. Eap45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. J Biol Chem. 2005;280:19600–19606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008688/GM/NIGMS NIH HHS/United States
- T32 GM007215/GM/NIGMS NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- R01 GM088151/GM/NIGMS NIH HHS/United States
- R01 GM110567/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials