Health outcomes of a high fructose intake: the importance of physical activity - PubMed (original) (raw)
Review
. 2019 Jul;597(14):3561-3571.
doi: 10.1113/JP278246. Epub 2019 Jun 9.
Affiliations
- PMID: 31116420
- PMCID: PMC6851848
- DOI: 10.1113/JP278246
Review
Health outcomes of a high fructose intake: the importance of physical activity
Luc Tappy et al. J Physiol. 2019 Jul.
Abstract
Fructose metabolism is generally held to occur essentially in cells of the small bowel, the liver, and the kidneys expressing fructolytic enzymes (fructokinase, aldolase B and a triokinase). In these cells, fructose uptake and fructolysis are unregulated processes, resulting in the generation of intracellular triose phosphates proportionate to fructose intake. Triose phosphates are then processed into lactate, glucose and fatty acids to serve as metabolic substrates in other cells of the body. With small oral loads, fructose is mainly metabolized in the small bowel, while with larger loads fructose reaches the portal circulation and is largely extracted by the liver. A small portion, however, escapes liver extraction and is metabolized either in the kidneys or in other tissues through yet unspecified pathways. In sedentary subjects, consumption of a fructose-rich diet for several days stimulates hepatic de novo lipogenesis, increases intrahepatic fat and blood triglyceride concentrations, and impairs insulin effects on hepatic glucose production. All these effects can be prevented when high fructose intake is associated with increased levels of physical activity. There is also evidence that, during exercise, fructose carbons are efficiently transferred to skeletal muscle as glucose and lactate to be used for energy production. Glucose and lactate formed from fructose can also contribute to the re-synthesis of muscle glycogen after exercise. We therefore propose that the deleterious health effects of fructose are tightly related to an imbalance between fructose energy intake on one hand, and whole-body energy output related to a low physical activity on the other hand.
Keywords: de novo lipogenesis; exercise metabolism; exercise recovery; gluconeogenesis; lactate production.
© 2019 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures
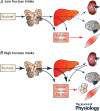
Figure 1. overview of fructose disposal pathways according to the amount of fructose ingested
Rodent studies indicate that with small oral loads (A), almost all ingested fructose is taken up in small bowel enterocytes to be released into the blood as glucose, lactate and various other metabolites. Under such conditions, portal fructose concentration, and hepatic and systemic fructose metabolism are very low. With larger fructose loads (B), intestinal fructose uptake is most likely saturated, and fructose is delivered into the hepatic portal blood, from which it is largely extracted by the liver. Under such conditions, both the gut and the liver release fructose carbons as glucose, lactate and triglyceride‐rich lipoproteins into the systemic circulation. A portion (ca 15% of a 30 g fructose load), however, escapes gut and hepatic uptake and reaches the systemic circulation. In humans, the maximal fructose load being taken up by the gut, and the relation between total fructose intake and systemic fructose appearance, remain still unknown. TRL‐TG: triglycerides in triglyceride‐rich lipoproteins.
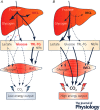
Figure 2. Proposed modulation of fructose metabolism by total energy output
Fructose's metabolic effects may be largely dependent on the balance between fructose intake and whole‐body energy output. Hepatic fructose uptake is essentially proportional to portal fructosaemia, i.e. to dietary fructose intake. Intrahepatic fructolysis is not regulated by insulin or intracellular energy status, and hence intrahepatic production of triose phosphates is proportional to fructose intake. At high fructose intake, the effects of this large triose phosphate flux vary according to extrahepatic energy output. When total energy output is low (A), hepatic triose phosphate production exceeds hepatic energy need. Triose phosphates are first channelled into lactate and glucose synthesis to be released into the bloodstream. Since glucose and lactate utilization rates in resting muscles are low, blood glucose, lactate and insulin concentration tend to increase, and prevent a further increase in hepatic glucose and lactate release. The excess triose phosphates are then channelled into liver glycogen and intrahepatocellular lipid storage, and triglyceride‐rich lipoprotein secretion. This may in the long term lead to hepatic insulin resistance, non‐alcoholic fatty liver disease and dyslipidaemia. When total energy output is high (B), as during physical exercise, hepatic glucose production increases as a result of hyperglucagonaemia, and glucose and lactate uptake increase to meet the increased muscle energy needs. Release of fructose carbons as blood glucose and lactate proceeds at a high rate, while its storage as liver glycogen and intrahepatocellular lipids or its release in triglyceride‐rich lipoproteins remains quantitatively low. According to this model, which remains in part hypothetical, adverse health effects of dietary fructose would appear only when fructose intake chronically exceeds the capacity of the liver to release lactate and glucose for the periphery, i.e. mainly when there is a mismatch between fructose intake and muscle energy output. CO2, carbon dioxide; IHCL, intrahepatic cellular lipids; IMCL, intramuscular cellular lipids; NEFA, non‐esterified fatty acids; TRL‐TG, triglycerides in triglyceride‐rich lipoproteins.
Similar articles
- Fructose and metabolic health: governed by hepatic glycogen status?
Hengist A, Koumanov F, Gonzalez JT. Hengist A, et al. J Physiol. 2019 Jul;597(14):3573-3585. doi: 10.1113/JP277767. Epub 2019 Apr 21. J Physiol. 2019. PMID: 30950506 Free PMC article. Review. - Metabolism of sugars: A window to the regulation of glucose and lipid homeostasis by splanchnic organs.
Tappy L. Tappy L. Clin Nutr. 2021 Apr;40(4):1691-1698. doi: 10.1016/j.clnu.2020.12.022. Epub 2020 Dec 21. Clin Nutr. 2021. PMID: 33413911 Review. - Does fructose consumption contribute to non-alcoholic fatty liver disease?
Tappy L, Lê KA. Tappy L, et al. Clin Res Hepatol Gastroenterol. 2012 Dec;36(6):554-60. doi: 10.1016/j.clinre.2012.06.005. Epub 2012 Jul 12. Clin Res Hepatol Gastroenterol. 2012. PMID: 22795319 Review. - Fructose metabolism and noncommunicable diseases: recent findings and new research perspectives.
Tappy L. Tappy L. Curr Opin Clin Nutr Metab Care. 2018 May;21(3):214-222. doi: 10.1097/MCO.0000000000000460. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29406418 Review. - Zinc inhibition of hepatic fructose metabolism in rats.
Coyle P, Tichelman E, Pauw R, Philcox J, Rofe A. Coyle P, et al. Biol Trace Elem Res. 2003 Apr;92(1):41-54. doi: 10.1385/bter:92:1:41. Biol Trace Elem Res. 2003. PMID: 12721403
Cited by
- Dose-response association between sugar- and artificially sweetened beverage consumption and the risk of metabolic syndrome: a meta-analysis of population-based epidemiological studies.
Zhang X, Li X, Liu L, Hong F, Zhao H, Chen L, Zhang J, Jiang Y, Zhang J, Luo P. Zhang X, et al. Public Health Nutr. 2021 Aug;24(12):3892-3904. doi: 10.1017/S1368980020003614. Epub 2020 Oct 28. Public Health Nutr. 2021. PMID: 33109289 Free PMC article. - Fructose Metabolism in Cancer.
Krause N, Wegner A. Krause N, et al. Cells. 2020 Dec 8;9(12):2635. doi: 10.3390/cells9122635. Cells. 2020. PMID: 33302403 Free PMC article. Review. - ALDOC- and ENO2- driven glucose metabolism sustains 3D tumor spheroids growth regardless of nutrient environmental conditions: a multi-omics analysis.
De Vitis C, Battaglia AM, Pallocca M, Santamaria G, Mimmi MC, Sacco A, De Nicola F, Gaspari M, Salvati V, Ascenzi F, Bruschini S, Esposito A, Ricci G, Sperandio E, Massacci A, Prestagiacomo LE, Vecchione A, Ricci A, Sciacchitano S, Salerno G, French D, Aversa I, Cereda C, Fanciulli M, Chiaradonna F, Solito E, Cuda G, Costanzo F, Ciliberto G, Mancini R, Biamonte F. De Vitis C, et al. J Exp Clin Cancer Res. 2023 Mar 22;42(1):69. doi: 10.1186/s13046-023-02641-0. J Exp Clin Cancer Res. 2023. PMID: 36945054 Free PMC article. - Effects of High Dietary Carbohydrate and Lipid Intake on the Lifespan of C. elegans.
Franco-Juárez B, Gómez-Manzo S, Hernández-Ochoa B, Cárdenas-Rodríguez N, Arreguin-Espinosa R, Pérez de la Cruz V, Ortega-Cuellar D. Franco-Juárez B, et al. Cells. 2021 Sep 8;10(9):2359. doi: 10.3390/cells10092359. Cells. 2021. PMID: 34572007 Free PMC article. Review. - Interindividual Variability in Postprandial Plasma Fructose Patterns in Adults.
Gladding M, Shen X, Snyder MP, Havel PJ, Adams SH. Gladding M, et al. Nutrients. 2024 Sep 13;16(18):3079. doi: 10.3390/nu16183079. Nutrients. 2024. PMID: 39339679 Free PMC article.
References
- Abdelmalek MF, Lazo M, Horska A, Bonekamp S, Lipkin EW, Balasubramanyam A, Bantle JP, Johnson RJ, Diehl AM & Clark JM (2012). Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 56, 952–960. - PMC - PubMed
- Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP & Jequier E (1988). Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am J Clin Nutr 48, 240–247. - PubMed
- Adopo E, Peronnet F, Massicotte D, Brisson GR & Hillaire‐Marcel C (1994). Respective oxidation of exogenous glucose and fructose given in the same drink during exercise. J Appl Physiol 76, 1014–1019. - PubMed
- Ahlborg G & Bjorkman O (1990). Splanchnic and muscle fructose metabolism during and after exercise. J Appl Physiol 69, 1244–1251. - PubMed
- Bantle JP, Raatz SK, Thomas W & Georgopoulos A (2000). Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr 72, 1128–1134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical