Early Growth Response 1 Deficiency Protects the Host against Pseudomonas aeruginosa Lung Infection - PubMed (original) (raw)
Early Growth Response 1 Deficiency Protects the Host against Pseudomonas aeruginosa Lung Infection
Zheng Pang et al. Infect Immun. 2019.
Abstract
Pseudomonas aeruginosa is an opportunistic pathogen that is a common cause of nosocomial infections. The molecular mechanisms governing immune responses to P. aeruginosa infection remain incompletely defined. Early growth response 1 (Egr-1) is a zinc-finger transcription factor that controls inflammatory responses. Here, we characterized the role of Egr-1 in host defense against P. aeruginosa infection in a mouse model of acute bacterial pneumonia. Egr-1 expression was rapidly and transiently induced in response to P. aeruginosa infection. Egr-1-deficient mice displayed decreased mortality, reduced levels of proinflammatory cytokines (tumor necrosis factor [TNF], interleukin-1β [IL-1β], IL-6, IL-12, and IL-17), and enhanced bacterial clearance from the lung. Egr-1 deficiency caused diminished NF-κB activation in _P. aeruginosa_-infected macrophages independently of IκBα phosphorylation. A physical interaction between Egr-1 and NF-κB p65 was found in _P. aeruginosa_-infected macrophages, suggesting that Egr-1 could be required for assembly of heterodimeric transcription factors that direct synthesis of inflammatory mediators. Interestingly, Egr-1 deficiency had no impact on neutrophil recruitment in vivo due to its differential effects on chemokine production, which included diminished accumulation of KC (CXCL1), MIP2 (CXCL2), and IP-10 (CXCL10) and increased accumulation of LIX (CXCL5). Importantly, Egr-1-deficient macrophages and neutrophils displayed significant increases in nitric oxide production and bacterial killing ability that correlated with enhanced bacterial clearance in Egr-1-deficient mice. Together, these findings suggest that Egr-1 plays a detrimental role in host defense against P. aeruginosa acute lung infection by promoting systemic inflammation and negatively regulating the nitric oxide production that normally assists with bacterial clearance.
Keywords: Egr-1; Pseudomonas aeruginosa; inflammation.
Copyright © 2019 Pang et al.
Figures
FIG 1
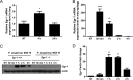
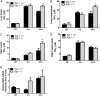
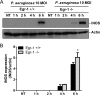
Egr-1 expression is induced in response to P. aeruginosa infection both in vivo and in vitro. Wild-type (+/+) and Egr-1-deficient (−/−) mice were intranasally infected with 1 × 109 CFU/mouse of P. aeruginosa 8821 for 4 h or 24 h or with an equivalent volume of saline solution as a control (NT). The total RNA extracted from lungs was reverse transcribed to cDNA and subjected to real-time quantitative PCR for Egr-1 gene expression. The gene expression was normalized to the HPRT housekeeping control gene (A) (n = 3 ± SEM; *, P < 0.05). BMMs were infected with P. aeruginosa strain 8821 at an MOI of 10 for 30 min, 1 h, 2 h, or 4 h or left untreated (NT). Total RNA isolated from these cells was reverse transcribed to cDNA and subjected to real-time quantitative PCR for Egr-1 gene expression. The Egr-1 mRNA levels were normalized to endogenous control HPRT (B) (n = 3 ± SEM; **, P < 0.01; ****, P < 0.0001). Cell lysates were subjected to Western blotting for Egr-1 protein expression, and actin was used as a loading control. Blots are representative of three independent experiments (C). Densitometry analysis of Egr-1 protein levels was normalized to actin, and data are presented as fold change (D) (n = 3 ± SEM; **, P < 0.01).
FIG 2
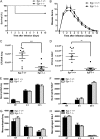
Egr-1 deficiency decreases mortality and enhances bacterial clearance but has no effect on neutrophil recruitment during P. aeruginosa infection in vivo. Wild-type (+/+) and Egr-1-deficient (−/−) mice were intranasally infected with 1 × 109 CFU/mouse of P. aeruginosa 8821 or an equivalent volume of saline solution as a control (NT). For survival study, mice were monitored daily up to 10 days (A), and the disease scores were calculated daily (B) (n = 10 ± SEM; *, P < 0.05; **, P < 0.01). To determine bacterial clearance, the bacterial burden in the lung (C) and BALF (D) was assessed at 24 h (n = 9; **, P < 0.01; ***, P < 0.001). For immune cell recruitment studies, lung tissues and BALF were collected at 4 h or 24 h postinfection. The numbers of neutrophils (E and F) and macrophages (G and H) that infiltrated into the lung (E and G) and BALF (F and H) were determined by flow cytometry analysis at 4 h and 24 h. A total of 5 × 104 cells from one lung or BALF sample was analyzed on a flow cytometer, and data are presented as cell numbers (n = 5).
FIG 3
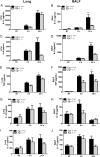
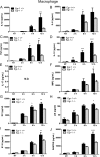
Egr-1-deficient mice displayed impaired proinflammatory cytokine production following P. aeruginosa lung infection. Wild-type (+/+) and Egr-1-deficient (−/−) mice were intranasally infected with 1 × 109 CFU/mouse of P. aeruginosa 8821 for 4 h or 24 h or with an equivalent volume of saline solution as a control (NT). Mice are sacrificed after infection time points. Lung tissues (A, C, G, E, and I) and BALF (B, D, F, H, and J) were collected for detection of IL-1β (A and B), IL-6 (C and D), TNF (E and F), IL-12 (G and H), and IL-17 (I and J) (n = 7 to 9 ± SEM; *, P < 0.05; **, P < 0.01; ****, P < 0.0001).
FIG 4
Egr-1 differentially regulates chemokine production following P. aeruginosa infection in vivo. Wild-type (+/+) and Egr-1-deficient (−/−) mice were intranasally infected with 1 × 109 CFU/mouse of P. aeruginosa 8821 for 4 h or 24 h or with an equivalent volume of saline solution as a control (NT). Mice are sacrificed after infection time points. Lung tissues (A, C, G, E, and I) and BALF (B, D, F, H, and J) were collected for detection of MIP2 (A and B), KC (C and D), LIX (E and F), IP-10 (G and H), and RANTES (I and J) (n = 7 to 9 ± SEM; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
FIG 5
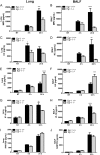
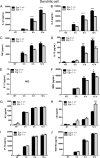
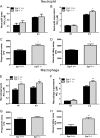
Egr-1-deficient BMMs display impaired proinflammatory cytokine and chemokine production following P. aeruginosa infection. Wild-type (+/+) and Egr-1-deficient (−/−) BMMs were infected with P. aeruginosa 8821 at an MOI of 10 for 3 h, 6 h, or 12 h or were left untreated (NT). Cell supernatants were collected for the determination of IL-1β (A), IL-6 (B), TNF (C), IL-12 (D), IL-17 (E), MIP2 (F), KC (G), LIX (H), IP-10 (I), and RANTES (J) secretion by ELISA (n = 3 ± SEM; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; N.D., not detected).
FIG 6
Egr-1-deficient BMDCs have increased LIX production following P. aeruginosa infection. Wild-type (+/+) and Egr-1-deficient (−/−) BMDCs were infected with P. aeruginosa strain 8821 at an MOI of 10 for 3 h, 6 h, or 12 h or were left untreated (NT). Cell supernatants were collected for the determination of IL-1β (A), IL-6 (B), TNF (C), IL-12 (D), IL-17 (E), MIP2 (F), KC (G), LIX (H), IP-10 (I), and RANTES (J) secretion by ELISA (n = 3 ± SEM; *, P < 0.05; **, P < 0.01; ****, P < 0.0001; N.D., not detected).
FIG 7
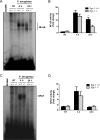
Egr-1 deficiency results in impaired NF-κB activation following P. aeruginosa infection in vivo. Wild-type (+/+) and Egr-1-deficient (−/−) mice were intranasally infected with 1 × 109 CFU/mouse of P. aeruginosa 8821 for 4 h or 24 h or with an equivalent volume of saline solution as a control (NT). Mice are sacrificed after infection time points. Nuclear proteins were extracted and subjected to EMSA by incubation with 32P-labeled NF-κB (A) and NFAT (C) DNA probes. Scan densitometry was performed for analysis of NF-κB (B) and NFAT (D) activation, and data are expressed as fold change versus wild-type untreated lung (n = 6 ± SEM; *, P < 0.05).
FIG 8
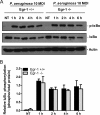
Egr-1 has no effect on IκBα phosphorylation in macrophages in response to P. aeruginosa infection. Wild-type (+/+) and Egr-1-deficient (−/−) BMMs were infected with P. aeruginosa 8821 at an MOI of 10 for 1 h, 2 h, 4 h, or 6 h or were left untreated (NT). Cell lysates were subjected to Western blotting for determining phosphorylated and total levels of IκBα, as well as of actin as a loading control. Blots are representative of three independent experiments (A). Densitometry analysis of phosphorylated IκBα was normalized to total IκBα (B) (n = 3 ± SEM).
FIG 9
Egr-1 physically interacts with NF-κB p65 in macrophages upon P. aeruginosa infection. Wild-type (+/+) and Egr-1-deficient (−/−) BMMs were infected with P. aeruginosa 8821 at an MOI of 10 for 1 h or left untreated (NT). Cell lysates were subjected to immunoprecipitation (IP) using anti-Egr-1 (A) or anti-p65 (B) antibody followed by Western blotting for Egr-1 or p65. Mouse or rabbit IgG was used as a control. Blots are representative of three independent experiments (n = 3). IB, immunoblotting.
FIG 10
Egr-1-deficient mice show enhanced nitric oxide production and upregulated iNOS mRNA expression following P. aeruginosa lung infection. Wild-type (+/+) and Egr-1-deficient (−/−) mice were intranasally infected with 1 × 109 CFU/mouse of P. aeruginosa 8821 for 4 h or 24 h or with an equivalent volume of saline solution as a control (NT). Mice are sacrificed after infection time points. The NO2− levels in lung lysates (A), lung supernatants (B), BALF lysates (C), and BALF supernatants (D) were assessed using a Griess reagent kit (n = 7 to 9 ± SEM; *, P < 0.05; **, P < 0.01). The total RNA extracted from lungs was reverse transcribed to cDNA and subjected to real-time quantitative PCR for iNOS gene expression. The gene expression was normalized to the HPRT housekeeping control gene (E) (n = 3 ± SEM; *, P < 0.05).
FIG 11
Egr-1-deficient BMMs display upregulated iNOS protein expression during P. aeruginosa infection. Wild-type (+/+) and Egr-1-deficient (−/−) BMMs were infected with P. aeruginosa 8821 at an MOI of 10 for 1 h, 2 h, 4 h, or 6 h or were left untreated (NT). Cell lysates were subjected to Western blotting for determining the protein levels of iNOS and actin as a loading control. Blots are representative of three independent experiments (A). Densitometry analysis of iNOS expression levels was normalized to actin (B) (n = 3 ± SEM; *, P < 0.05).
FIG 12
Egr-1 deficiency leads to increased nitric oxide production and enhanced bacterial intracellular levels in neutrophils and macrophages in response to P. aeruginosa infection. Wild-type (+/+) and Egr-1-deficient (−/−) neutrophils (A to D) and BMMs (E to H) were infected with P. aeruginosa 8821 for various durations. The NO2− levels in cell lysates (A and E) and supernatants (B and F) were determined at 6 h. The P. aeruginosa neutrophils or macrophages were infected for 1 h and lysed for phagocytosis assay (C and G). The CFU data represent the number of internalized bacteria within 1 h (n = 6 ± SEM; *, P < 0.05). The P. aeruginosa-infected neutrophils or macrophages were infected for 3 h and lysed for bacterial killing assay (D and H). The intracellular killing efficiency was calculated as the number of CFU after 1 h minus the number of CFU after 3 h of infection (1 h CFU − 3 h CFU) (n = 6 ± SEM; *, P < 0.05; **, P < 0.01).
FIG 13
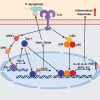
Schematic representation of Egr-1-regulated host defense against P. aeruginosa infection. Egr-1 negatively regulates nitric oxide production by suppressing iNOS gene expression and promotes inflammatory cytokine production by physically interacting with NF-κB p65 during P. aeruginosa infection.
Similar articles
- Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Véliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C. Véliz Rodriguez T, et al. Infect Immun. 2012 Jan;80(1):100-9. doi: 10.1128/IAI.05695-11. Epub 2011 Oct 24. Infect Immun. 2012. PMID: 22025515 Free PMC article. - Signal transducer and activator of transcription 4 (STAT4), but not IL-12 contributes to Pseudomonas aeruginosa-induced lung inflammation in mice.
O'Sullivan R, Carrigan SO, Marshall JS, Lin TJ. O'Sullivan R, et al. Immunobiology. 2008;213(6):469-79. doi: 10.1016/j.imbio.2007.11.007. Epub 2008 Jan 2. Immunobiology. 2008. PMID: 18514749 - Mice Lacking γδ T Cells Exhibit Impaired Clearance of Pseudomonas aeruginosa Lung Infection and Excessive Production of Inflammatory Cytokines.
Omar T, Ziltener P, Chamberlain E, Cheng Z, Johnston B. Omar T, et al. Infect Immun. 2020 May 20;88(6):e00171-20. doi: 10.1128/IAI.00171-20. Print 2020 May 20. Infect Immun. 2020. PMID: 32229615 Free PMC article. - The role of IL-1β in Pseudomonas aeruginosa in lung infection.
Wonnenberg B, Bischoff M, Beisswenger C, Dinh T, Bals R, Singh B, Tschernig T. Wonnenberg B, et al. Cell Tissue Res. 2016 May;364(2):225-9. doi: 10.1007/s00441-016-2387-9. Epub 2016 Mar 17. Cell Tissue Res. 2016. PMID: 26984603 Review. - Airway immunometabolites fuel Pseudomonas aeruginosa infection.
Riquelme SA, Prince A. Riquelme SA, et al. Respir Res. 2020 Dec 10;21(1):326. doi: 10.1186/s12931-020-01591-x. Respir Res. 2020. PMID: 33302964 Free PMC article. Review.
Cited by
- A multiomics approach to identify host-microbe alterations associated with infection severity in diabetic foot infections: a pilot study.
Radzieta M, Sadeghpour-Heravi F, Peters TJ, Hu H, Vickery K, Jeffries T, Dickson HG, Schwarzer S, Jensen SO, Malone M. Radzieta M, et al. NPJ Biofilms Microbiomes. 2021 Mar 22;7(1):29. doi: 10.1038/s41522-021-00202-x. NPJ Biofilms Microbiomes. 2021. PMID: 33753735 Free PMC article. - P. aeruginosa type III and type VI secretion systems modulate early response gene expression in type II pneumocytes in vitro.
Sen-Kilic E, Huckaby AB, Damron FH, Barbier M. Sen-Kilic E, et al. BMC Genomics. 2022 May 4;23(1):345. doi: 10.1186/s12864-022-08554-0. BMC Genomics. 2022. PMID: 35508983 Free PMC article. - Early Growth Response 1 Suppresses Macrophage Phagocytosis by Inhibiting NRF2 Activation Through Upregulation of Autophagy During Pseudomonas aeruginosa Infection.
Pang Z, Xu Y, Zhu Q. Pang Z, et al. Front Cell Infect Microbiol. 2022 Jan 12;11:773665. doi: 10.3389/fcimb.2021.773665. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35096638 Free PMC article. - Effects of Egr1 on pancreatic acinar intracellular trypsinogen activation and the associated ceRNA network.
Gao B, Zhang X, Xue D, Zhang W. Gao B, et al. Mol Med Rep. 2020 Sep;22(3):2496-2506. doi: 10.3892/mmr.2020.11316. Epub 2020 Jul 9. Mol Med Rep. 2020. PMID: 32705196 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases