Cardiomyocyte-Specific Snrk Prevents Inflammation in the Heart - PubMed (original) (raw)
. 2019 Nov 19;8(22):e012792.
doi: 10.1161/JAHA.119.012792. Epub 2019 Nov 13.
Stephanie M Cossette 1, Qiulun Lu 2, Shreya R Chowdhury 3, Leanne M Harmann 4, Ankan Gupta 1, Andrew D Spearman 5, Dmitry L Sonin 6, Michelle Bordas 1, Suresh N Kumar 7, Amy Y Pan 8, Pippa M Simpson 8, Jennifer L Strande 4, Erin Bishop 3, Ming-Hui Zou 2, Ramani Ramchandran 1 3
Affiliations
- PMID: 31718444
- PMCID: PMC6915262
- DOI: 10.1161/JAHA.119.012792
Cardiomyocyte-Specific Snrk Prevents Inflammation in the Heart
Karthikeyan Thirugnanam et al. J Am Heart Assoc. 2019.
Abstract
Background The SNRK (sucrose-nonfermenting-related kinase) enzyme is critical for cardiac function. However, the underlying cause for heart failure observed in Snrk cardiac conditional knockout mouse is unknown. Methods and Results Previously, 6-month adult mice knocked out for Snrk in cardiomyocytes (CMs) displayed left ventricular dysfunction. Here, 4-month adult mice, on angiotensin II (Ang II) infusion, show rapid decline in cardiac systolic function, which leads to heart failure and death in 2 weeks. These mice showed increased expression of nuclear factor κ light chain enhancer of activated B cells (NF-κB), inflammatory signaling proteins, proinflammatory proteins in the heart, and fibrosis. Interestingly, under Ang II infusion, mice knocked out for Snrk in endothelial cells did not show significant systolic or diastolic dysfunction. Although an NF-κB inflammation signaling pathway was increased in Snrk knockout endothelial cells, this did not lead to fibrosis or mortality. In hearts of adult mice knocked out for Snrk in CMs, we also observed NF-κB pathway activation in CMs, and an increased presence of Mac2+ macrophages was observed in basal and Ang II-infused states. In vitro analysis of Snrk knockdown HL-1 CMs revealed similar upregulation of the NF-κB signaling proteins and proinflammatory proteins that was exacerbated on Ang II treatment. The Ang II-induced NF-κB pathway-mediated proinflammatory effects were mediated in part through protein kinase B or AKT, wherein AKT inhibition restored the proinflammatory signaling protein levels to baseline in Snrk knockdown HL-1 CMs. Conclusions During heart failure, SNRK acts as a cardiomyocyte-specific repressor of cardiac inflammation and fibrosis.
Keywords: NF‐kB; cardiac hypertrophy; cardiomyocyte; endothelial cell; fibrosis; heart failure; inflammation.
Figures
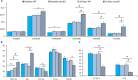
Figure 1
Angiotensin II (Ang
II
) induces cardiac failure in 2 weeks in Snrk cmc
KO
mice. A through C, Echocardiogram results for baseline 4‐month‐old Snrk wild‐type (
WT
) mice and cardiac‐specific knockout (Snrk cmc
KO
) mice with Ang
II
infused for 14 days into Snrk
WT
mice and 14 days into cardiac‐specific knockout (Snrk cmc
KO
) mice as described before.15 All data were normalized to body weight (
BW
) and presented as such. The parameters analyzed are interventricular septum thickness at end‐diastole (
IVS
d/
BW
), left ventricular internal dimension at end‐diastole (
LVID
d/
BW
), left ventricular posterior wall thickness at end‐diastole (
LVPW
d/
BW
), left ventricular internal dimension at end‐systole (
LVID
s/
BW
), end‐diastole volume (
EDV
/
BW
), end‐systolic volume (
ESV
/
BW
), isovolumic relaxation time (
IVRT
), peak velocity of early diastolic transmitral flow (E), early diastolic mitral annular velocity (e′), pulmonary acceleration rate (
PAT
), ejection time (
ET
), ejection fraction (
EF
), fractional shortening (
FS
). Results are presented as mean±
SEM
(*P<0.05, # P<0.01). The statistical comparison for P value was done by comparing Snrk
WT
vs Snrk cmc
KO
, Snrk
WT
vs Snrk
WT
‐Ang
II
, Snrk cmc
KO
vs Snrk cmc
KO
‐Ang
II
, and Snrk
WT
‐Ang
II
vs Snrk cmc
KO
‐Ang
II
(n=6 for the WT group and n=3 for Snrk cmc
KO
and the Ang
II
–induced experimental group).
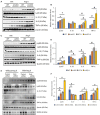
Figure 2
Angiotensin II (Ang
II
)–infused hearts from Snrk conditional knockout mice and Snrk knockdown cardiomyocytes (
CM
s) show higher levels of proinflammatory response. A and B, Hearts from Snrk cmc
KO
and (C and D) Snrk ec
KO
mice were assessed for pro‐ and anti‐inflammatory signaling by immunoblotting. Both knockout mouse groups were analyzed under vehicle‐treated and Ang
II
–induced conditions similar to wild‐type (WT) control mice.
NF
‐κB p‐p65,
IL
‐6, and
TNF
‐α were assessed for proinflammatory signaling, and
IL
‐10 was assessed for anti‐inflammatory signaling. Results are presented as mean±
SEM
(*P<0.05 and # P<0.01). The statistical comparison for P value was done by comparing Snrk
WT
vs Snrk cmc
KO
, Snrk
WT
vs Snrk
WT
‐Ang
II
, Snrk cmc
KO
vs Snrk cmc
KO
‐Ang
II
and Snrk
WT
‐Ang
II
vs Snrk cmc
KO
‐Ang
II
(n=3 animals in each experimental group). E and F,
HL
‐1 cardiomyocyte cells were treated with Snrk small interfering (si)
RNA
with and without Ang
II
and assessed for pro‐ and anti‐inflammatory signaling. Results are presented as mean±
SEM
(*P<0.05 and # P<0.01). The statistical comparison for P value was done by comparing control si
RNA
vs Snrk si
RNA
, control si
RNA
vs control si
RNA
‐Ang
II
, Snrk si
RNA
vs Snrk si
RNA
‐Ang
II
, and control si
RNA
‐Ang
II
vs Snrk si
RNA
‐Ang
II
(n=3 in each experimental group). cmcKO indicates cardiomyocyte knockout; ecKO, endothelial cell knockout; SEM, standard error of the mean.
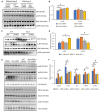
Figure 3
Angiotensin II (Ang
II
)–infused hearts from Snrk cmc
KO
and Snrk knockdown cardiomyocytes (
CM
s) show changes in
pAKT
and
pERK
signaling pathways. A and B, Immunoblotting analysis of
HL
‐1 cardiomyocytes were treated with Snrk small interfering (si)
RNA
with and without Ang
II
(1 μmol/L) for 24 hours. Results are presented as mean±
SEM
(*P<0.05). Statistical comparison for P value was done by comparing control si
RNA
vs Snrk si
RNA
, control si
RNA
vs control si
RNA
‐Ang
II
, Snrk si
RNA
vs Snrk si
RNA
‐Ang
II
, and control si
RNA
‐Ang
II
vs Snrk si
RNA
‐Ang
II
(n=3 in each experimental group). C and D, Snrk cmc
KO
mouse hearts were assessed for phosphorylated forms of
AKT
and
ERK
signaling by immunoblotting, KO mouse hearts were analyzed under vehicle‐treated and Ang
II
–induced conditions, wild‐type (WT) mice were used as controls for both conditions. Results are presented as mean±
SEM
(*P<0.05 and # P<0.01). The statistical comparison for P value was done by comparing Snrk
WT
vs Snrk cmc
KO
, Snrk
WT
vs Snrk
WT
‐Ang
II
, Snrk cmc
KO
vs Snrk cmc
KO
‐Ang
II
, and Snrk
WT
‐Ang
II
vs Snrk cmc
KO
‐Ang
II
(n=3 animals in each experimental group). E and F, Briefly,
HL
‐1 cardiomyocyte cells were subjected to Snrk si
RNA
transfection and treated with and without
AKT
inhibitor
LY
294002 (10 μmol/L) for 24 hours and assessed for phosphorylated
AKT
and the proinflammatory
NF
‐κB p‐p65,
IL
‐6, and
TNF
‐α and anti‐inflammatory
IL
‐10 signaling.
DMSO
‐treated cells served as control. Results are presented as mean±
SEM
(*P<0.05 and # P<0.01 compared with respective treatment groups; n=3 in each experimental group). cmcKO indicates cardiomyocyte knockout; SEM, standard error of the mean.
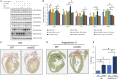
Figure 4
AKT
inhibition under angiotensin II (Ang
II
)–stimulated conditions in cardiomyocytes (
CM
s) attenuate inflammation. A,
HL
‐1
CM
s were transfected with control and Snrk small interfering (si)
RNA
, studied with and without Ang
II
(1 μmol/L) and/or
AKT
inhibitor
LY
294002 (10 μmol/L), and assessed for phosphorylated‐
AKT
(p‐
AKT
) and proinflammatory markers
NF
‐κB p‐p65,
IL
‐6, and
TNF
‐α. Untreated cells served as control. Results are presented as mean±
SEM
(*P<0.05 and # P<0.01 vs respective control si
RNA
‐treated cells; n=3 in each experimental group). B, Quantification of blots from sample in A. C through E, Snrk cmc
KO
hearts were analyzed for fibrosis using Sirius red staining, which showed an exacerbated fibrotic environment determined by increased accumulation of collagen in the hearts. The mice were treated with vehicle control (C) and Ang II (D). Significant increases in the accumulation of collagen in the Snrk cmc
KO
mice were observed compared with wild type (WT); on stimulation with Ang
II
the collagen deposition increases further. Results are presented as mean±
SEM
(# P<0.01). The statistical comparison for P value was done by comparing Snrk
WT
vs Snrk cmc
KO
, Snrk
WT
vs Snrk
WT
–Ang
II
, Snrk cmc
KO
vs Snrk cmc
KO
–Ang
II
, and Snrk
WT
–Ang
II
vs Snrk cmc
KO
–Ang
II
(n=3 animals in each experimental group). cmcKO indicates cardiomyocyte knockout; SEM, standard error of the mean; veh, vehicle.
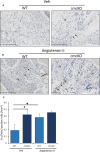
Figure 5
SNRK
enhances angiotensin II (Ang
II
)–induced inflammation in Snrk cmc
KO
hearts. A, Wild‐type (WT) and Snrk cardiac knockout mice (cmc
KO
) were treated with vehicle control and Ang II (B). Increased infiltration of macrophages in the Snrk cmc
KO
mice was observed compared with WT on stimulation with Ang
II
, and macrophage numbers further increased. Scale bars are 250 μm for Snrk
WT
, Snrk cmc
KO
, and Snrk
WT
‐Ang
II
and 100 μm for Snrk cmc
KO
‐Ang
II
. C, Results are presented as mean±
SEM
(*P<0.05 and # P<0.01). The statistical comparison for P value was done by comparing Snrk
WT
vs Snrk cmc
KO
, Snrk
WT
vs Snrk
WT
–Ang
II
, Snrk cmc
KO
vs Snrk cmc
KO
–Ang
II
, and Snrk
WT
–Ang
II
vs Snrk cmc
KO
–Ang
II
(n=3 animals in each experimental group). SEM indicates standard error of the mean; veh, vehicle.
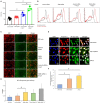
Figure 6
NF
‐κB transcription and expression in
HL
‐1 cardiomyocytes. A and B, Flow cytometry analysis for detection of
HL
‐1 cells positive for the expression of
NF
‐κB p‐p65. Results are presented as mean±
SEM
(# P<0.01). The statistical comparison for P value was done by comparing control small interfering (si)
RNA
vs Snrk si
RNA
, control si
RNA
vs Control si
RNA
–angiotensin II (Ang
II
), Snrk si
RNA
vs Snrk si
RNA
–Ang
II
, and control si
RNA
–Ang
II
vs Snrk si
RNA
–Ang
II
(n=3 per each experimental group). C and D, Reporter vector gene assay for the analysis of transcriptional activation of
NF
‐κB.
NF
‐κB vector was tagged with
GFP
protein,
RFP
acted as positive control for the assessment of infection efficiency. Scale bars are at 200 μm. Results are presented as mean±
SEM
(*P<0.05). The statistical comparison for P value was done by comparing control si
RNA
vs Snrk si
RNA
, control si
RNA
vs control si
RNA
–Ang
II
, Snrk si
RNA
vs Snrk si
RNA
–Ang
II
, and control si
RNA
–Ang
II
vs Snrk si
RNA
–Ang
II
(n=12 in each experimental group). E and F,
HL
‐1 cells were treated with
AKT
inhibitor/Ang
II
, where Ang
II
–treated
HL
‐1 cells show more
NF
‐κB p‐p65. The resulting colocalizations were quantified using ImageJ software and represented as graphs. Scale bars are at 1 μm. Results are presented as mean±
SEM
(# P<0.01 vs untreated control cells; n=3 in each experimental group). GFP indicates green fluorescent protein; MFI, Mean Fluorescence Intensity; RFP, red fluorescent protein; SEM, standard error of the mean.
Similar articles
- Sucrose Nonfermenting-Related Kinase Enzyme-Mediated Rho-Associated Kinase Signaling is Responsible for Cardiac Function.
Cossette SM, Bhute VJ, Bao X, Harmann LM, Horswill MA, Sinha I, Gastonguay A, Pooya S, Bordas M, Kumar SN, Mirza SP, Palecek SP, Strande JL, Ramchandran R. Cossette SM, et al. Circ Cardiovasc Genet. 2016 Dec;9(6):474-486. doi: 10.1161/CIRCGENETICS.116.001515. Epub 2016 Oct 25. Circ Cardiovasc Genet. 2016. PMID: 27780848 Free PMC article. - Insulin-Like Growth Factor 1 Receptor Deficiency Alleviates Angiotensin II-Induced Cardiac Fibrosis Through the Protein Kinase B/Extracellular Signal-Regulated Kinase/Nuclear Factor-κB Pathway.
Zhu J, Li Q, Sun Y, Zhang S, Pan R, Xie Y, Chen J, Shi L, Chen Y, Sun Z, Zhang L. Zhu J, et al. J Am Heart Assoc. 2023 Sep 19;12(18):e029631. doi: 10.1161/JAHA.123.029631. Epub 2023 Sep 18. J Am Heart Assoc. 2023. PMID: 37721135 Free PMC article. - Inflammation and NLRP3 Inflammasome Activation Initiated in Response to Pressure Overload by Ca2+/Calmodulin-Dependent Protein Kinase II δ Signaling in Cardiomyocytes Are Essential for Adverse Cardiac Remodeling.
Suetomi T, Willeford A, Brand CS, Cho Y, Ross RS, Miyamoto S, Brown JH. Suetomi T, et al. Circulation. 2018 Nov 27;138(22):2530-2544. doi: 10.1161/CIRCULATIONAHA.118.034621. Circulation. 2018. PMID: 30571348 Free PMC article. - Pathophysiology of Angiotensin II-Mediated Hypertension, Cardiac Hypertrophy, and Failure: A Perspective from Macrophages.
Carter K, Shah E, Waite J, Rana D, Zhao ZQ. Carter K, et al. Cells. 2024 Dec 4;13(23):2001. doi: 10.3390/cells13232001. Cells. 2024. PMID: 39682749 Free PMC article. Review. - Overview of pyroptosis mechanism and in-depth analysis of cardiomyocyte pyroptosis mediated by NF-κB pathway in heart failure.
Zhang Z, Yang Z, Wang S, Wang X, Mao J. Zhang Z, et al. Biomed Pharmacother. 2024 Oct;179:117367. doi: 10.1016/j.biopha.2024.117367. Epub 2024 Aug 29. Biomed Pharmacother. 2024. PMID: 39214011 Review.
Cited by
- Exenatide inhibits NF-κB and attenuates ER stress in diabetic cardiomyocyte models.
Fu Z, Mui D, Zhu H, Zhang Y. Fu Z, et al. Aging (Albany NY). 2020 May 11;12(9):8640-8651. doi: 10.18632/aging.103181. Epub 2020 May 11. Aging (Albany NY). 2020. PMID: 32392536 Free PMC article. - Navigating the Maze of Kinases: CaMK-like Family Protein Kinases and Their Role in Atherosclerosis.
Teuwen JTJ, van der Vorst EPC, Maas SL. Teuwen JTJ, et al. Int J Mol Sci. 2024 Jun 5;25(11):6213. doi: 10.3390/ijms25116213. Int J Mol Sci. 2024. PMID: 38892400 Free PMC article. Review. - Histone Acetyltransferase p300 Inhibitor Improves Coronary Flow Reserve in SIRT3 (Sirtuin 3) Knockout Mice.
Su H, Zeng H, He X, Zhu SH, Chen JX. Su H, et al. J Am Heart Assoc. 2020 Sep 15;9(18):e017176. doi: 10.1161/JAHA.120.017176. Epub 2020 Aug 31. J Am Heart Assoc. 2020. PMID: 32865093 Free PMC article. - SNRK: a metabolic regulator with multifaceted role in development and disease.
Thirugnanam K, Ramchandran R. Thirugnanam K, et al. Vessel Plus. 2020;4:26. Epub 2020 Aug 21. Vessel Plus. 2020. PMID: 32968716 Free PMC article. - Human bone marrow mesenchymal stem cell-derived extracellular vesicles reduce inflammation and pyroptosis in acute kidney injury via miR-223-3p/HDAC2/SNRK.
Xie Z, Tang J, Chen Z, Wei L, Chen J, Liu Q. Xie Z, et al. Inflamm Res. 2023 Mar;72(3):553-576. doi: 10.1007/s00011-022-01653-4. Epub 2023 Jan 14. Inflamm Res. 2023. PMID: 36640195 Free PMC article.
References
- Giamouzis G, Kalogeropoulos A, Georgiopoulou V, Laskar S, Smith AL, Dunbar S, Triposkiadis F, Butler J. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17:54–75. - PubMed
- Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. - PubMed
- Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119:159–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA213022/CA/NCI NIH HHS/United States
- R13 HL147501/HL/NHLBI NIH HHS/United States
- R01 HL142287/HL/NHLBI NIH HHS/United States
- R01 HL080499/HL/NHLBI NIH HHS/United States
- R01 HL123338/HL/NHLBI NIH HHS/United States
- R01 HL134932/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous