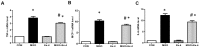Attenuation of diabetic kidney injury in DPP4-deficient rats; role of GLP-1 on the suppression of AGE formation by inducing glyoxalase 1 - PubMed (original) (raw)
Attenuation of diabetic kidney injury in DPP4-deficient rats; role of GLP-1 on the suppression of AGE formation by inducing glyoxalase 1
Mithun Kumer Sarker et al. Aging (Albany NY). 2020.
Abstract
Dipeptidyl peptidase 4 (DPP4) inactivates incretin hormone glucagon-like peptide-1. DPP4 inhibitors may exert beneficial effects on diabetic nephropathy (DN) independently of glycemic control; however, the mechanisms underlying are not fully understood. Here, we investigated the mechanisms of the beneficial effects of DPP4 inhibition on DN using DPP4-deficient (DPP4-def) rats and rat mesangial cells.Blood glucose and HbA1c significantly increased by streptozotocin (STZ) and no differences were between WT-STZ and DPP4-def-STZ. The albumin level in urine decreased significantly and the albumin/creatinine ratio decreased slightly in DPP4-def-STZ. The glomerular volume in DPP4-def-STZ significantly decreased compared with that of WT-STZ. Advanced glycation end products formation, receptor for AGE (RAGE) protein expression, and its downstream inflammatory cytokines and fibrotic factors in kidney tissue, were significantly suppressed in the DPP4-def-STZ compared to the WT-STZ with increasing glyoxalase-1 (GLO-1) expression responsible for the detoxification of methylglyoxal (MGO). In vitro, exendin-4 suppressed MGO-induced AGEs production by enhancing the expression of GLO-1 and nuclear factor-erythroid 2 p45 subunit-related factor 2, resulting in decreasing pro-inflammatory cytokine levels. This effect was abolished by GLO-1 siRNA.Our data suggest that endogenously increased GLP-1 in DPP4-deficient rats contributes to the attenuation of DN partially by regulating AGEs formation via upregulation of GLO-1 expression.
Keywords: advanced glycation end products; diabetic nephropathy; dipeptidyl peptidase 4; glucagon-like peptide-1; glyoxalase-1.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors declare that there is no conflicts of interest.
Figures
Figure 1
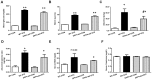
DPP4 deficiency attenuates albuminuria in STZ-induced diabetic rats. Both wild-type and DPP4-deficient rats were administered with IP injection at 30 mg/kg/day STZ three times. All samples were collected and evaluated as described in Materials and Methods. (A) Blood glucose level after 4 h fasting, (B) HbA1c level, (C) Albuminuria level, (D) Albumin/creatinine ratio. (E) Serum BUN level, (F) Serum creatinine level. WT-CON: wild-type control, WT-STZ: wild-type-STZ, DPP4-def-CON: DPP4-deficient control, DPP4-def-STZ: DPP4-deficient-STZ. Data are shown as the means ± SEM. *p < 0.05, ** p < 0.01 and WT-CON, #p < 0.05 and WT-STZ, n = 7–8 per group.
Figure 2
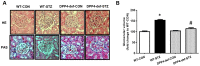
DPP4 deficiency recovers the structure of glomerulus impaired by STZ. Kidney samples were collected at 42 days, since over 300 mg/dL of blood glucose after STZ injection as described in the Materials and Methods. The glomerular volume was measured using the ImageJ software for at least 15 images from each kidney section. (A) Representative image of glomerulus by H&E staining and by PAS staining, (B) Glomerular volume. WT-CON: wild-type control, WT-STZ: wild-type-STZ, DPP4-def-CON: DPP4-deficient control, DPP4-def-STZ: DPP4-deficient-STZ. Data are shown as the means ± SEM. *p< 0.05 and WT-CON, #p < 0.05 and WT-STZ, n = 7–8 per group.
Figure 3
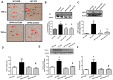
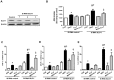
The expression of inflammatory cytokines and fibrotic factors are reduced in STZ-induced diabetic rats. (A) The kidney tissues were fixed in formalin and then subjected to immunofluorescence detection of TNF-α (arrow heads pointing to dark-brown dots indicating TNF-α expression). n = 5 per group, (B) IL6 protein level (C) MCP1 protein level (D) TGF-β mRNA level, (E) TGF-β protein level with a representative blot, (F) Fibronectin (FN) mRNA level in kidney tissues. Data are shown as the means ± SEM *p < 0.05 vs. WT-CON; #p < 0.05 vs. WT-STZ. n = 5–8 per group.
Figure 4
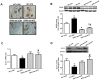
Increased of AGE formation, RAGE and GLO-1 expression are inhibited in the kidney of DPP4 deficient diabetic rats. Kidney samples were collected at 42 days, since over 300 mg/dL of blood glucose after STZ injection as described in Materials and Methods section. AGEs formation was evaluated using antibody against AGEs in the kidney section. Brown color indicates AGEs formation in staining. (A) AGEs formation, (B) RAGE protein level with representative blot (C) GLO1 mRNA level (D) GLO-1 protein level with a representative blot in tissues. WT-CON: wild-type control, WT-STZ: wild-type-STZ, DPP4-def-CON: DPP4-deficient control, DPP4-def-STZ: DPP4-deficient-STZ. Data are shown as the means ± SEM. *p < 0.05 and WT-CON, #p < 0.05 and WT-STZ, n = 7–8 per group.
Figure 5
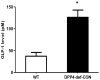
Circulating plasma GLP-1 level is increased in DPP4-deficient rats. Plasma GLP-1 concentration was measured using rat-specific GLP-1 ELISA kit within 3 h after collecting blood from wild-type and DPP4-deficient rats at 8 weeks of age. Data are shown as the means ± SEM. *p < 0.05 and WT, n = 4–6 per group.
Figure 6
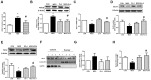
Ex-4 treatment reduces MGO-induced AGEs formation and RAGE expression by upregulating GLO-1 enzyme and recovers the decrease in MGO–induced GLO-1 expression in rat mesangial cells. Rat mesangial cells were treated either with 1 mM MGO, 10 nM Ex-4, or both for 10 h after synchronization with 1% fetal bovine serum for 13-16 h. AGEs formation was measured as described in Materials and Methods. (A) AGEs formation, (B) RAGE protein level with representative blot, (C) GLO-1 mRNA level, and (D) GLO-1 protein level with a representative blot (E) Nrf-2 protein level with a representative blot in total protein extracts, (F) Representative blot of Nrf-2 protein in cytosol and nuclear fractions in rat mesangial cells. 1: CON; 2: MGO; 3: Ex-4; 4: MGO + Ex-4 (G) Nrf-2 protein level in cytosol fraction. (H) Nrf-2 protein level in nuclear fraction. Data are shown as the means ± SEM. *p < 0.05 and CON, #p < 0.05 and MGO, n = 4–7.
Figure 7
Ex-4 treatment reduces MGO-induced inflammatory cytokine expression in rat mesangial cells. Rat mesangial cells were treated either with 1 mM MGO, 10 nM Ex-4, or both for 10 h after synchronization with 1% fetal bovine serum for 13-16 h. (A) TNF-α mRNA level, (B) MCP-1 mRNA level, (C) IL6 mRNA level in rat mesangial cells. Data are shown as the means ± SEM. *p < 0.05 and CON, #p < 0.05 and MGO, n = 3–4.
Figure 8
AGEs formation and inflammatory cytokines are further increased in the knockdown of GLO-1. Rat mesangial cells were transfected either with siRNA control or siRNA GLO-1 and, then, treated with 0.75 mM MGO and 10 nM Ex-4 for 4 h. (A) siRNA GLO-1 transfection efficacy. (B) AGEs formation level in the knockdown of GLO-1 and mRNA expression levels of inflammatory cytokines including (C) TNF-α (D) MCP-1, and (E) IL-6. Data are shown as the means ± SEM. *p < 0.05 vs. siRNA CON, #p < 0.05 vs. siRNA CON + MGO, †p < 0.05 vs. siRNA GLO-1 + CON, ‡p < 0.05 vs. siRNA CON + MGO + Ex-4. n = 5–6.
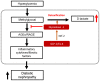
Figure 9
Schematic diagram in STZ-induced diabetic nephropathy showing GLP-1/Ex-4 increases detoxification of methylglyoxal (MGO) through the regulation of glyoxalase-1. Hyperglycemia-induced MGO accumulation under diabetic condition activates the AGEs-RAGE signaling pathway, which results in diabetic nephropathy through upregulation of the expression of inflammatory cytokines and fibrotic factors. In contrast, GLP-1/Ex-4 enhances detoxification of MGO, producing D-lactate through the regulation of glyoxalase-1 expression. GLP-1: Glucagon like peptide-1, Ex-4: Exendin-4, Nrf-2: Nuclear factor-erythroid 2 p45 subunit-related factor-2, AGEs: Advanced glycation end products, RAGE: Receptor AGE.
Similar articles
- The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats.
Zhang MH, Feng L, Zhu MM, Gu JF, Jiang J, Cheng XD, Ding SM, Wu C, Jia XB. Zhang MH, et al. J Ethnopharmacol. 2014;151(1):591-600. doi: 10.1016/j.jep.2013.11.015. Epub 2013 Nov 21. J Ethnopharmacol. 2014. PMID: 24269777 - Hesperetin ameliorates diabetic nephropathy in rats by activating Nrf2/ARE/glyoxalase 1 pathway.
Chen YJ, Kong L, Tang ZZ, Zhang YM, Liu Y, Wang TY, Liu YW. Chen YJ, et al. Biomed Pharmacother. 2019 Mar;111:1166-1175. doi: 10.1016/j.biopha.2019.01.030. Epub 2019 Jan 12. Biomed Pharmacother. 2019. PMID: 30841430 - Phenethyl isothiocyanate attenuates diabetic nephropathy via modulation of glycative/oxidative/inflammatory signaling in diabetic rats.
Eisa NH, Khodir AE, El-Sherbiny M, Elsherbiny NM, Said E. Eisa NH, et al. Biomed Pharmacother. 2021 Oct;142:111666. doi: 10.1016/j.biopha.2021.111666. Epub 2021 Jun 29. Biomed Pharmacother. 2021. PMID: 34215478 Retracted. - Can Targeting the Incretin Pathway Dampen RAGE-Mediated Events in Diabetic Nephropathy?
Sourris KC, Yao H, Jerums G, Cooper ME, Ekinci EI, Coughlan MT. Sourris KC, et al. Curr Drug Targets. 2016;17(11):1252-64. doi: 10.2174/1389450116666150722141418. Curr Drug Targets. 2016. PMID: 26201485 Review. - The Role of Glyoxalase in Glycation and Carbonyl Stress Induced Metabolic Disorders.
Saeed M, Kausar MA, Singh R, Siddiqui AJ, Akhter A. Saeed M, et al. Curr Protein Pept Sci. 2020;21(9):846-859. doi: 10.2174/1389203721666200505101734. Curr Protein Pept Sci. 2020. PMID: 32368974 Review.
Cited by
- Therapeutic Potential of Phlorotannin-Rich Ecklonia cava Extract on Methylglyoxal-Induced Diabetic Nephropathy in In Vitro Model.
Cho CH, Lee CJ, Kim MG, Ryu B, Je JG, Kim Y, Lee SH. Cho CH, et al. Mar Drugs. 2022 May 27;20(6):355. doi: 10.3390/md20060355. Mar Drugs. 2022. PMID: 35736158 Free PMC article. - Glyoxalase 1 Confers Susceptibility to Schizophrenia: From Genetic Variants to Phenotypes of Neural Function.
Yin J, Ma G, Luo S, Luo X, He B, Liang C, Zuo X, Xu X, Chen Q, Xiong S, Tan Z, Fu J, Lv D, Dai Z, Wen X, Zhu D, Ye X, Lin Z, Lin J, Li Y, Chen W, Luo Z, Li K, Wang Y. Yin J, et al. Front Mol Neurosci. 2021 Nov 1;14:739526. doi: 10.3389/fnmol.2021.739526. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34790095 Free PMC article. - Improvement effect of gemigliptin on salivary gland dysfunction in exogenous methylglyoxal-injected rats.
Jung WK, Park SB, Yu HY, Kim J. Jung WK, et al. Heliyon. 2024 Apr 9;10(8):e29362. doi: 10.1016/j.heliyon.2024.e29362. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38628768 Free PMC article. - Glyoxalase System in the Progression of Skin Aging and Skin Malignancies.
Yumnam S, Subedi L, Kim SY. Yumnam S, et al. Int J Mol Sci. 2020 Dec 30;22(1):310. doi: 10.3390/ijms22010310. Int J Mol Sci. 2020. PMID: 33396745 Free PMC article. Review. - Gemigliptin Improves Salivary Gland Dysfunction in D-Galactose-Injected Aging Rats.
Jung WK, Park SB, Yu HY, Kim J. Jung WK, et al. Pharmaceutics. 2023 Dec 26;16(1):35. doi: 10.3390/pharmaceutics16010035. Pharmaceutics. 2023. PMID: 38258046 Free PMC article.
References
- Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, De Mey JG, Schalkwijk CG. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010; 53:989–1000. 10.1007/s00125-010-1677-0 - DOI - PMC - PubMed
- Hanssen NM, Scheijen JL, Jorsal A, Parving HH, Tarnow L, Rossing P, Stehouwer CD, Schalkwijk CG. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease in Individuals With Type 1 Diabetes: A 12-Year Follow-up Study. Diabetes. 2017; 66:2278–83. 10.2337/db16-1578 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous