High-fat diet-induced dysbiosis mediates MCP-1/CCR2 axis-dependent M2 macrophage polarization and promotes intestinal adenoma-adenocarcinoma sequence - PubMed (original) (raw)
. 2020 Feb;24(4):2648-2662.
doi: 10.1111/jcmm.14984. Epub 2020 Jan 19.
Affiliations
- PMID: 31957197
- PMCID: PMC7028862
- DOI: 10.1111/jcmm.14984
High-fat diet-induced dysbiosis mediates MCP-1/CCR2 axis-dependent M2 macrophage polarization and promotes intestinal adenoma-adenocarcinoma sequence
Tianyu Liu et al. J Cell Mol Med. 2020 Feb.
Abstract
High-fat diet (HFD) is a well-known risk factor for gut microbiota dysbiosis and colorectal cancer (CRC). However, evidence relating HFD, gut microbiota and carcinogenesis is limited. Our study aimed to demonstrate that HFD-induced gut dysbiosis promoted intestinal adenoma-adenocarcinoma sequence. In clinical study, we found that HFD increased the incidence of advanced colorectal neoplasia (AN). The expression of monocyte chemoattractant protein 1 (MCP-1), CC chemokine receptor 2 (CCR2) and CD163 in CRC patients with HFD was significantly higher than that in CRC patients with normal diet. When it comes to the Apcmin/+ mice, HFD consumption could induce gut dysbiosis and promote intestinal carcinogenesis, accompanying with activation of MCP-1/CCR2 axis that recruited and polarized M2 tumour-associated macrophages. Interestingly, transfer of faecal microbiota from HFD-fed mice to another batch of Apcmin/+ mice in the absence of HFD could also enhance carcinogenesis without significant body weight gain and induced MCP-1/CCR2 axis activation. HFD-induced dysbiosis could also be transmitted. Meanwhile, antibiotics cocktail treatment was sufficient to inhibit HFD-induced carcinogenesis, indicating the vital role of dysbiosis in cancer development. Conclusively, these data indicated that HFD-induced dysbiosis accelerated intestinal adenoma-adenocarcinoma sequence through activation of MCP-1/CCR2 axis, which would provide new insight into better understanding of the mechanisms and prevention for HFD-related CRC.
Keywords: MCP-1/CCR2 axis; gut microbiota; high-fat diet; intestinal carcinogenesis; tumour-associated macrophages.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
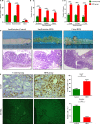
High‐fat diet up‐regulated the expression of MCP‐1 and CCR2 in human colorectal cancer tissues. A‐C, Immunohistochemistry analysis of CRC tissues. Scale bar: 50 μm. ***P < .001. CRC, colorectal cancer. Control, n = 15; HFD, n = 15
Figure 2
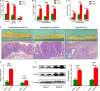
High‐fat diet accelerated intestinal adenoma‐adenocarcinoma sequence. A‐C, Tumour numbers of the HFD group and control group. D, The representative gross and histological appearance of intestinal tumours from the HFD group and control group were shown. E, Small intestinal sections from HFD‐treated and untreated Apcmin/+ mice were stained with Ki‐67 and TUNEL. Scale bar: 50 μm. **P < .01, ***P < .001. HFD, high‐fat diet. Control, n = 7; HFD, n = 8. HFD + Abx, n = 8
Figure 3
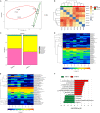
High‐fat diet‐induced gut dysbiosis during intestinal carcinogenesis. A, Beta‐diversity analysis showed a clear difference. B, Principal component analysis (PCA) of faecal bacteria in two groups. C, Ace index reflected the species richness of faecal samples. D‐F, The microbiota community composition between HFD group and control group was different at phylum, genus and species levels. G, The concentrations of SCFAs (Acetate and Butyrate) in caecal contents were significantly decreased after HFD treatment. H, Analysis of gut microbiota at different taxonomy levels and key bacteria changes during carcinogenesis. *P < .05. Control, n = 7; HFD, n = 8
Figure 4
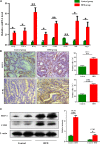
High‐fat diet‐induced dysbiosis activated MCP‐1/CCR2 axis. A, The inflammatory factors and TAMs mRNA levels in the small intestinal tumours. B‐C, Immunohistochemistry staining and Western blot results showed that the protein expression levels of MCP‐1 and CCR2 in intestinal tumours were significantly increased in HFD group. Scale bar: 50 μm. *P < .05, **P < .01, ***P < .001
Figure 5
A‐C, Immunofluorescence assay suggested that the activated MCP‐1/CCR2 axis promoted M2 tumour‐associated macrophages recruitment and polarization. Scale bar: 50μm
Figure 6
Faecal microbiota from high‐fat diet‐treated Apcmin/+ mice activated MCP‐1/CCR2 axis and accelerated carcinogenesis. A‐C, Tumour numbers in both groups after FMT. D, The representative gross and histological appearance of intestinal tumours in both groups. E‐F, The expression levels of MCP‐1 and CCR2 in small intestine tumours of FMT‐H group were significantly higher than those in FMT‐C group. Scale bar: 50 μm. *P < .05, **P < .01, ***P < .001. FMT‐C, n = 4; FMT‐H, n = 4
Figure 7
High‐fat diet‐induced dysbiosis could be transmitted to another batch of Apcmin/+ mice. A‐B, Principal component analysis (PCA) and beta‐diversity analysis showed clear difference in faecal microbiota clustering between FMT‐H group and FMT‐C group. C, Differences in microbial community composition between two groups at phylum level. D‐F, Heatmap and LEfSe results showed mice receiving the faecal microbiota from HFD‐fed donors had increased levels of opportunistic pathogens and decreased beneficial bacteria. HFD, high‐fat diet. FMT‐C (transplantation of faecal microbiota from control group to a new batch of recipient _Apcmin/+_mice). FMT‐H (transplantation of faecal microbiota from HFD group to a new batch of recipient _Apcmin/+_mice). FMT‐C, n = 4; FMT‐H, n = 4
Figure 8
Schematic overview of high‐fat diet‐induced gut dysbiosis on intestinal carcinogenesis. HFD intake significantly altered the gut microbial composition and caused dysbiosis in Apcmin/+ mice. Disrupted intestinal barrier favoured the bacterial translocation. The dysbiosis activated the MCP‐1/CCR2 signalling axis to recruit and polarize M2 TAMs. In addition, these changes increased the release of inflammatory mediators and also reduced the SCFAs production. These cancer‐promoting processes promoted tumour cell proliferation and inhibited apoptosis to facilitate colorectal neoplastic progression. Antibiotics cocktail treatment could reverse HFD‐induced intestinal carcinogenesis, whereas transfer of faecal microbiota from HFD‐fed mice to another batch of Apcmin/+ mice in the absence of HFD could activate MCP‐1/CCR2 axis and promote the development of adenoma‐adenocarcinoma sequence. HFD, high‐fat diet; FMT, faecal microbiota transplantation; MCP‐1, monocyte chemoattractant protein 1; CCR2, CC chemokine receptor 2; TAMs, tumour‐associated macrophages; SCFAs, short‐chain fatty acids; IL‐1β, interleukin‐1β; TNF‐α, tumour necrosis factor‐α; IFN‐γ, interferon‐γ
Similar articles
- High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites.
Yang J, Wei H, Zhou Y, Szeto CH, Li C, Lin Y, Coker OO, Lau HCH, Chan AWH, Sung JJY, Yu J. Yang J, et al. Gastroenterology. 2022 Jan;162(1):135-149.e2. doi: 10.1053/j.gastro.2021.08.041. Epub 2021 Aug 27. Gastroenterology. 2022. PMID: 34461052 - Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis.
Wang S, Dong W, Liu L, Xu M, Wang Y, Liu T, Zhang Y, Wang B, Cao H. Wang S, et al. Mol Carcinog. 2019 Jul;58(7):1155-1167. doi: 10.1002/mc.22999. Epub 2019 Mar 3. Mol Carcinog. 2019. PMID: 30828892 Free PMC article. - High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity.
Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C, Salinas-Riester G, Böck A, Alpert C, Blaut M, Polson SC, Brandl L, Kirchner T, Greten FR, Polson SW, Arkan MC. Schulz MD, et al. Nature. 2014 Oct 23;514(7523):508-12. doi: 10.1038/nature13398. Epub 2014 Aug 31. Nature. 2014. PMID: 25174708 Free PMC article. - Intestinal microbiota and its association with colon cancer and red/processed meat consumption.
Abu-Ghazaleh N, Chua WJ, Gopalan V. Abu-Ghazaleh N, et al. J Gastroenterol Hepatol. 2021 Jan;36(1):75-88. doi: 10.1111/jgh.15042. Epub 2020 Apr 3. J Gastroenterol Hepatol. 2021. PMID: 32198788 Review. - Metabolic Interaction Between Host and the Gut Microbiota During High-Fat Diet-Induced Colorectal Cancer.
Lee C, Lee S, Yoo W. Lee C, et al. J Microbiol. 2024 Mar;62(3):153-165. doi: 10.1007/s12275-024-00123-2. Epub 2024 Apr 16. J Microbiol. 2024. PMID: 38625645 Review.
Cited by
- Transcriptomic and Proteomic Study on the High-Fat Diet Combined With AOM/DSS-Induced Adenomatous Polyps in Mice.
Guo C, Xu Y, Han X, Liu X, Xie R, Cheng Z, Fu X. Guo C, et al. Front Oncol. 2021 Aug 26;11:736225. doi: 10.3389/fonc.2021.736225. eCollection 2021. Front Oncol. 2021. PMID: 34513713 Free PMC article. - Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis.
Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Nishikawa H, et al. Int J Mol Sci. 2020 Jul 24;21(15):5254. doi: 10.3390/ijms21155254. Int J Mol Sci. 2020. PMID: 32722100 Free PMC article. Review. - Failed Induction of the TH1 System in TH2 Dominant Patients: The Cancer-Permissive Immune Macroenvironment.
Yanuck SF. Yanuck SF. Integr Med (Encinitas). 2024 May;23(2):24-35. Integr Med (Encinitas). 2024. PMID: 38911450 Free PMC article. - Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy.
Zhu R, Lang T, Yan W, Zhu X, Huang X, Yin Q, Li Y. Zhu R, et al. Adv Sci (Weinh). 2021 Mar 9;8(10):2003542. doi: 10.1002/advs.202003542. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026439 Free PMC article. Review. - Dietary-Induced Bacterial Metabolites Reduce Inflammation and Inflammation-Associated Cancer via Vitamin D Pathway.
O'Mahony C, Clooney A, Clarke SF, Aguilera M, Gavin A, Simnica D, Ahern M, Fanning A, Stanley M, Rubio RC, Patterson E, Marques T, Wall R, Houston A, Mahmoud A, Bennett MW, Stanton C, Claesson MJ, Cotter PD, Shanahan F, Joyce SA, Melgar S. O'Mahony C, et al. Int J Mol Sci. 2023 Jan 18;24(3):1864. doi: 10.3390/ijms24031864. Int J Mol Sci. 2023. PMID: 36768196 Free PMC article.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
- Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210‐215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous