Down-regulation of SLC35C1 induces colon cancer through over-activating Wnt pathway - PubMed (original) (raw)
. 2020 Mar;24(5):3079-3090.
doi: 10.1111/jcmm.14969. Epub 2020 Jan 21.
Affiliations
- PMID: 31961998
- PMCID: PMC7077602
- DOI: 10.1111/jcmm.14969
Down-regulation of SLC35C1 induces colon cancer through over-activating Wnt pathway
Minzi Deng et al. J Cell Mol Med. 2020 Mar.
Abstract
The canonical Wnt signalling pathway is a critical pathway involved in the proliferation of cells. It has been well-established that it plays the central role during colorectal carcinogenesis and development. Yet the exact molecular mechanism of how the canonical Wnt pathway is fine-tuned remains elusive. We found that SLC35C1, a GDP-fucose transporter, negatively regulates the Wnt signalling pathway. We show here that SLC35C1 is reduced in all colon cancer by both immunohistochemistry images and TCGA data, whereas β-catenin is increased. Down-regulation of SLC35C1 is also detected by real-time PCR in stage 3 and stage 4 colorectal cancer tissues. Moreover, analysing the TCGA database with cBioPortal reveals the negative correlation of SLC35C1 mRNA level to the expression of β-catenin. Reduced SLC35C1 significantly promotes cell proliferation and colony formation of HEK293 cells. Meanwhile, in HEK293 cells silencing SLC35C1 activates canonical Wnt pathway, whereas overexpressing SLC35C1 inhibits it. Consistently, the reduction of SLC35C1 in HEK293 cells also elevated the mRNA level of Wnt target genes C-myc, Axin2 and Cyclin D1, as well as the secretion of Wnt3a. In conclusion, we identified SLC35C1 as a negative regulator of the Wnt signalling pathway in colon cancer. Decreased SLC35C1 may cause over-activation of Wnt signalling in colorectal cancer.
Keywords: SLC35C1; Wnt signalling; colon cancer; fucosylation; β-catenin.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
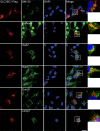
Subcellular distribution of SLC35C1HEK293 cells were transfected with Flag‐tagged SLC35C1. 48 h later, immunofluorescence was performed with antibodies against Flag tag, GM130 (a marker for Golgi apparatus), calnexin (a marker for ER), Rab5 (a marker for early endosome), Rab7 (marker for late endosome), Lamp1 (a marker for lysosome) and TOM20 (a marker for mitochondria). Confocal images demonstrated that SLC35C1 colocalizes with GM130, calnexin, Rab5 and Rab7, indicating SLC35C1 locates at the Golgi apparatus, ER, and early and late endosome. No colocalization SLC35C1 with Lamp1 or TOM20 was found, suggesting SLC35C1 is not in lysosome or mitochondria. Bar = 10 μm
Figure 2
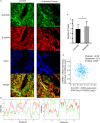
Distribution of SLC35C1 in tissue. A, Tissues from normal donor and stage 2 colon cancer patients were subjected to fluorescent immunohistochemistry with antibodies against β‐catenin (red) and SLC35C1 (green). The images show that β‐catenin does not coincide with SLC35C1 in both normal and cancer tissue. B, The colour profile shows the separated distribution of SLC35C1 and β‐catenin. Multichannel line profile analysis is done by RGB profiler plug‐in of ImageJ. Briefly, a random line is drawn through the green particles on the merged image, and the profile of both green channel and red channel along the selected line is plotted by the RGB profiler, with the X‐axis implies the distance on the line and the Y‐axis implies the fluorescent intensity. C, The intensity of SLC35C1 negatively correlates with that of β‐catenin. Six samples from the control group and 6 samples from the cancer group were included; for each sample, 3 random lines were selected and plotted for colour profiling. Based on the colour profile, the numbers of places where the two‐colour peaks coincide together or separates were counted (Mean ± SD, *P = .02, two‐tailed t test with Welches’ correction). D, TCGA data confirm the negative correlation between SLC35C1 and β‐catenin expression. Analysis of the TCGA database using cBioPortal revealed that the mRNA expression of SLC35C1 negatively correlates with the mRNA level of β‐catenin. mRNA level of SLC35C1 and β‐catenin was presented with log2 value
Figure 3
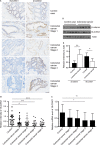
SLC35C1 is down‐regulated in colon cancer tissue. A, H&E staining shows decreased SLC35C1 and increased β‐catenin in colon cancer tissue. Tissue samples from healthy donors and colon cancer patients were subjected to immunohistochemistry assay. H&E staining revealed that SLC35C1 expression is significantly reduced at each stage of colon cancer where β‐catenin is increased. Twenty‐one healthy controls, 16 stage 1 samples, 19 stage 2 samples, 15 stage 3 samples and 24 stage 4 samples were included in the assay. B, Statistical results of Figure 1A (Mean ± SD, *P < .05, **P < .01, ***P < .001, one‐way ANOVA). The relative level of SLC35C1 was determined by calculating the average H&E intensity of each sample normalized to the average intensity of a randomly selected control sample. C, Real‐time PCR shows the mRNA level of SLC35C1 is decreased in stage 3 and stage 4 colorectal cancer. The expression of SLC35C1 is quantified by real‐time PCR and presented by the ratios between SLC35C1 and β‐actin transcripts for normalization (Mean ± SD, *P < .05, **P < .01, one‐way ANOVA). D, Western blot shows increased β‐catenin and reduced SLC35C1 level in colorectal cancer. Tissue samples were subjected to Western blot to compare the protein level of β‐catenin and SLC35C1 in healthy and colorectal cancer. β‐actin was used as the loading control. E, Statistical results of Figure 3D. (Mean ± SD, *P < .05, **P < .01, Student's t test.) The intensity of the bands was normalized to the intensity of matching actin bands
Figure 4
SLC35C1 is down‐regulated in colon cancer whereas β‐catenin is elevated. Colon adenocarcinoma (COAD) samples collected in TCGA base is analysed with web tool UALCAN. Results show that: A, SLC35C1 is significantly down‐regulated in primary colon cancer (P = .0004); B, SLC35C1 is significantly down‐regulated in all stages of colon cancer (P = .0002, 0.0003, 0.0057, 0.0075, respectively); C, β‐catenin is significantly down‐regulated in primary colon cancer (P < 1E‐12); D, β‐catenin is significantly down‐regulated in all stages of colon cancer (P = 9.42E‐10, P = 1.62E‐12, P = 5.52E‐14, respectively). **P < .01 and ***P < .001
Figure 5
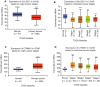
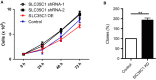
Silencing SLC35C1 promotes cell growth and clone formation. A, Control HEK293 cells and cells with SLC35C1 silenced or overexpressed were seeded in 6‐well plate. Each well started with 50 000 cells, and 4 time‐points were calculated. Each time‐point was prepared in triplicates. To calculate cells, wells were trypsinized and cell numbers were counted under a stereo microscope. Results show that cell proliferation was induced in response to SLC35C1 silencing with either shRNA constructs (*P < .05, **P < .01). Conversely, overexpressing SLC35C1 inhibits cell growth (**P < .05). SLC35C1 shRNA‐1 and SLC35C1shRNA‐2 indicate SLC35C1 silenced with two shRNA strands constructs; SLC35C1 OE indicates SLC35C1 overexpressed HEK293 cells. Data were presented as Mean ± SD. Statistic significance was analysed with two‐way ANOVA with multiple comparisons using GraphPad Prism7. B, Control HEK293 cells and cells with SLC35C1 silenced were subjected to colony formation assay. Silencing SLC35C1 significantly increased the number of colonies formed in soft agar (Mean ± SD, **P < .01)
Figure 6
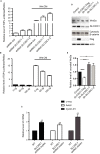
SLC35C1 negatively regulates Wnt pathway activity. A, TOPFLASH assay shows activation of the canonical Wnt pathway by silencing SLC35C1 in HEK293 cells. HEK293 cells were transfected with two different strands of shRNA. 48 h later, TOPFLASH assay was performed and revealed increased luciferase intensity after silencing SLC35C1 compared to the intensity in cells transfected with scrambled siRNA (shRNA ctrl), whereas stimulating with conditioned medium collected from L Wnt‐3A cells (Wnt CM) for 12 h significantly reversed the effect caused by SLC35C1 silence (Mean ± SD). B, TOPFLASH assay shows overexpressing SLC35C1 in HEK293 cells inhibits the canonical Wnt pathway in a concentration‐dependent manner. HEK293 cells were overexpressed with SLC35C1 (0 ng plasmid, 50 ng plasmid, 100 ng plasmid). 48 h later, TOPFLASH assay revealed decreased luciferase intensity, and this inhibition can be restored by Wnt CM stimulation for 12 h (Mean ± SD). C, Real‐time PCR shows increased mRNA level of Wnt target genes in SLC35C1 silenced HEK293 cells. Endogenous SLC35C1 gene in HEK293 cell was silenced using the shRNAs system. 48 h later, mRNA level of Wnt target genes, including C‐myc, Axin2 and Cyclin D1, was found to be elevated by real‐time PCR, suggesting that absence of SLC35C1 activates Wnt signalling pathway (Mean ± SD, *P < .05, **P < .01). D, Western blot shows silencing SLC35C1 alters the level of Wnt3a and β‐catenin in HEK293 cells. HEK293 cells were transfected with either exogenous SLC35C1 or shRNAs that silence endogenous SLC35C1. 48 h later, cells were lysed, and the culture medium was collected and centrifuged at 13 000 g to remove the debris. Cell lysates and centrifuged supernatants were then loaded for Western blot. Results showed that Wnt 3a level is reduced by overexpressed SLC35C1 but induced by silencing SLC35C. On the contrary, the amount of cytosolic β‐catenin is negatively associated with SLC35C1 level. E, Statistical results of Figure 6D (Mean ± SD, *P < .05, ***P < .001)
Similar articles
- MiR-34b inhibits the proliferation and promotes apoptosis in colon cancer cells by targeting Wnt/β-catenin signaling pathway.
Ye K, Xu C, Hui T. Ye K, et al. Biosci Rep. 2019 Oct 30;39(10):BSR20191799. doi: 10.1042/BSR20191799. Biosci Rep. 2019. PMID: 31467172 Free PMC article. Retracted. - Protein kinase C delta negatively modulates canonical Wnt pathway and cell proliferation in colon tumor cell lines.
Hernández-Maqueda JG, Luna-Ulloa LB, Santoyo-Ramos P, Castañeda-Patlán MC, Robles-Flores M. Hernández-Maqueda JG, et al. PLoS One. 2013;8(3):e58540. doi: 10.1371/journal.pone.0058540. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520519 Free PMC article. Clinical Trial. - Wnt3a Promotes the Vasculogenic Mimicry Formation of Colon Cancer via Wnt/β-Catenin Signaling.
Qi L, Song W, Liu Z, Zhao X, Cao W, Sun B. Qi L, et al. Int J Mol Sci. 2015 Aug 10;16(8):18564-79. doi: 10.3390/ijms160818564. Int J Mol Sci. 2015. PMID: 26266404 Free PMC article. - Wnt signaling in cell adhesion, development, and colon cancer.
Tejeda-Muñoz N, Mei KC. Tejeda-Muñoz N, et al. IUBMB Life. 2024 Jul;76(7):383-396. doi: 10.1002/iub.2806. Epub 2024 Jan 17. IUBMB Life. 2024. PMID: 38230869 Review. - Role of Wnt3a in the pathogenesis of cancer, current status and prospective.
Pashirzad M, Fiuji H, Khazei M, Moradi-Binabaj M, Ryzhikov M, Shabani M, Avan A, Hassanian SM. Pashirzad M, et al. Mol Biol Rep. 2019 Oct;46(5):5609-5616. doi: 10.1007/s11033-019-04895-4. Epub 2019 Jun 24. Mol Biol Rep. 2019. PMID: 31236761 Review.
Cited by
- The analysis of tumor-infiltrating immune cell and ceRNA networks in laryngeal squamous cell carcinoma.
Li D, Dong K, Su J, Xue H, Tian J, Wu Y, Wang J. Li D, et al. Medicine (Baltimore). 2022 Aug 5;101(31):e29555. doi: 10.1097/MD.0000000000029555. Medicine (Baltimore). 2022. PMID: 35945754 Free PMC article. - 11p11.12p12 duplication in a family with intellectual disability and craniofacial anomalies.
Chen X, Xu H, Shi W, Wang F, Xu F, Zhang Y, Gan J, Tian X, Chen B, Dai M. Chen X, et al. BMC Med Genomics. 2021 Apr 9;14(1):99. doi: 10.1186/s12920-021-00945-8. BMC Med Genomics. 2021. PMID: 33836758 Free PMC article. - Transcriptome Differences Suggest Novel Mechanisms for Intrauterine Growth Restriction Mediated Dysfunction in Small Intestine of Neonatal Piglets.
Huang S, Wu Z, Yuan X, Li N, Li T, Wang J, Levesque CL, Feng C. Huang S, et al. Front Physiol. 2020 Jun 23;11:561. doi: 10.3389/fphys.2020.00561. eCollection 2020. Front Physiol. 2020. PMID: 32655399 Free PMC article. - Systematic pan-cancer analysis identifies SLC35C1 as an immunological and prognostic biomarker.
Xie M, Wang F, Chen B, Wu Z, Chen C, Xu J. Xie M, et al. Sci Rep. 2023 Apr 1;13(1):5331. doi: 10.1038/s41598-023-32375-0. Sci Rep. 2023. PMID: 37005450 Free PMC article. - Triclosan activates c-Jun/miR-218-1-3p/SLC35C1 signaling to regulate cell viability, migration, invasion and inflammatory response of trophoblast cells in vitro.
Huo W, Wang Y, Chen T, Cao T, Zhang Y, Shi Z, Hou S. Huo W, et al. BMC Pregnancy Childbirth. 2022 Jun 6;22(1):470. doi: 10.1186/s12884-022-04791-z. BMC Pregnancy Childbirth. 2022. PMID: 35668364 Free PMC article.
References
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177‐193. - PubMed
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843‐850. - PubMed
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781‐810. - PubMed
- Bhanot P, Brink M, Samos CH, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225‐230. - PubMed
- Yang‐Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302‐1306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous