IRF5 Promotes Influenza Virus-Induced Inflammatory Responses in Human Induced Pluripotent Stem Cell-Derived Myeloid Cells and Murine Models - PubMed (original) (raw)
. 2020 Apr 16;94(9):e00121-20.
doi: 10.1128/JVI.00121-20. Print 2020 Apr 16.
Jessica L Forbester 1 2 3, Dannielle Wellington 2 5, Amy Yeung 3 6, Sandra Dimonte 4, Morgan Marsden 4, Lucy Chapman 4, Eve L Coomber 3, Charlotte Tolley 3, Emily Lees 3, Christine Hale 3, Simon Clare 3, Irina Udalova 7, Tao Dong 2 5, Gordon Dougan 3 6, Ian R Humphreys 4
Affiliations
- PMID: 32075938
- PMCID: PMC7163152
- DOI: 10.1128/JVI.00121-20
IRF5 Promotes Influenza Virus-Induced Inflammatory Responses in Human Induced Pluripotent Stem Cell-Derived Myeloid Cells and Murine Models
Jessica L Forbester et al. J Virol. 2020.
Abstract
Recognition of influenza A virus (IAV) by the innate immune system triggers pathways that restrict viral replication, activate innate immune cells, and regulate adaptive immunity. However, excessive innate immune activation can exaggerate disease. The pathways promoting excessive activation are incompletely understood, with limited experimental models to investigate the mechanisms driving influenza virus-induced inflammation in humans. Interferon regulatory factor 5 (IRF5) is a transcription factor that plays important roles in the induction of cytokines after viral sensing. In an in vivo model of IAV infection, IRF5 deficiency reduced IAV-driven immune pathology and associated inflammatory cytokine production, specifically reducing cytokine-producing myeloid cell populations in _Irf5_-/- mice but not impacting type 1 interferon (IFN) production or virus replication. Using cytometry by time of flight (CyTOF), we identified that human lung IRF5 expression was highest in cells of the myeloid lineage. To investigate the role of IRF5 in mediating human inflammatory responses by myeloid cells to IAV, we employed human-induced pluripotent stem cells (hIPSCs) with biallelic mutations in IRF5, demonstrating for the first time that induced pluripotent stem cell-derived dendritic cells (iPS-DCs) with biallelic mutations can be used to investigate the regulation of human virus-induced immune responses. Using this technology, we reveal that IRF5 deficiency in human DCs, or macrophages, corresponded with reduced virus-induced inflammatory cytokine production, with IRF5 acting downstream of Toll-like receptor 7 (TLR7) and, possibly, retinoic acid-inducible gene I (RIG-I) after viral sensing. Thus, IRF5 acts as a regulator of myeloid cell inflammatory cytokine production during IAV infection in mice and humans and drives immune-mediated viral pathogenesis independently of type 1 IFN and virus replication.IMPORTANCE The inflammatory response to influenza A virus (IAV) participates in infection control but contributes to disease severity. After viral detection, intracellular pathways are activated, initiating cytokine production, but these pathways are incompletely understood. We show that interferon regulatory factor 5 (IRF5) mediates IAV-induced inflammation and, in mice, drives pathology. This was independent of antiviral type 1 IFN and virus replication, implying that IRF5 could be specifically targeted to treat influenza virus-induced inflammation. We show for the first time that human iPSC technology can be exploited in genetic studies of virus-induced immune responses. Using this technology, we deleted IRF5 in human myeloid cells. These IRF5-deficient cells exhibited impaired influenza virus-induced cytokine production and revealed that IRF5 acts downstream of Toll-like receptor 7 and possibly retinoic acid-inducible gene I. Our data demonstrate the importance of IRF5 in influenza virus-induced inflammation, suggesting that genetic variation in the IRF5 gene may influence host susceptibility to viral diseases.
Keywords: IRF5; dendritic cells; iPSCs; inflammation; influenza.
Copyright © 2020 Forbester et al.
Figures
FIG 1
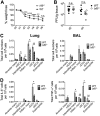
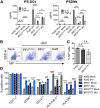
IRF5 alters cytokine responses to influenza A virus in a murine infection model. WT and _Irf5_−/− mice were infected intranasally with 3 × 103 A/X-31 influenza virus. (A) Inflammatory cytokine expression in BAL fluid was measured using multiplex assays 2, 4, and 7 days p.i. Data shown are the mean ± SEM using 7 WT and 5 _Irf5_−/− mice (day 2) or five mice per genotype (day 4 and day 7) and represent the results from two independent experiments. (B) IFN-α and IFN-β levels in BAL fluid measured by ELISA in Irf5−/− and WT naive and IAV-infected mice at 2 days p.i. Data shown are the mean ± SEM of the results from 3 to 6 mice per group at 2 days p.i.
FIG 2
IRF5 enhances influenza A virus-induced inflammatory response in a murine infection model. (A) Weight loss of WT and Irf5−/− mice was assessed over time, and comparable results were observed in 4 independent experiments, with 4 to 5 WT or _Irf5_−/− mice in each group per experiment. Data shown are the mean ± SEM. (B) Replication of virus in the lungs was quantified using a plaque assay. Data shown are the mean ± SEM using 7 WT and 5 _Irf5_−/− mice for day 2 and 5 mice of each genotype for day 4. (C) Recruitment of specific myeloid cell populations (mDCs, monocyte-derived DCs; cDCs, conventional DCs; pDCs, plasmacytoid DCs; Inflam. mon, inflammatory monocytes) in WT and _Irf5_−/− mice was assessed by flow cytometry 2 days p.i. Populations were defined by the following markers: alveolar macrophages (Alveolar macs), SiglecF+ CD11b+ CD64+ Ly6C−; mDCs, SiglecF− CD11b+ MHC-II+ CD11c+ CD64+ Ly6C+; interstitial macrophages, SiglecF− CD11b+ MHC-II+ CD11c− CD64+ Ly6C+; inflammatory monocytes, SiglecF− CD11b+ MHC-II− Ly6C+ CD64+; cDCs, MHC-II+ CD11c+ Ly6C−; pDCs, B220+ SiglecH+ MHC-IIlow CD11clow; and eosinophils, SiglecF+ CD11c− CD11b+ Ly6C−. Data shown are the mean ± SEM using 11 WT and 10 Irf5−/− mice from multiple replicates. (D) The total number of each individual myeloid cell population (unstimulated, ex vivo) positive for IL-6 and TNF-α expression was detected by flow cytometry, with data presented representing the mean total cell number per 105 cells of each cell type ± SEM. Data represent two experiments.
FIG 3
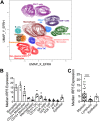
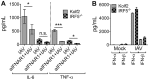
IRF5 expression in human lung cells. IRF5 expression by multiple cellular subsets derived from human lung tissue from independent donors was analyzed by CyTOF. (A) UMAP based on downsampled, concatenated files from lung samples from four donors using phenotypic markers. Post-UMAP analysis, populations (colored by cell type as identified by lung CyTOF) were defined via the following markers: CD4+ T cells, CD3+ CD4+ CD20−; CD8+ T cells, CD3+ CD20− CD8+; B cells, CD3− CD20+; NK cells, CD3− CD20− CD56+; CD14+ monocytes, CD16− CD11b+ CD14+ HLA-DR+; CD16+ monocytes, CD14− CD11b+ CD16+ HLA-DR+; macrophages, CD11b+ CD68+ HLA-DR+; pDCs, CD123+ CD11b+ HLA-DR+; CD141+ cDCs, CD11b+ HLA-DR+ CD1c− CD141+; CD1c+ cDCs, CD11b+ HLA-DR+ CD1c+ CD141−; eosinophils, Siglec8+ CD123−; and basophils, Siglec8+ CD123+. (B) Median IRF5 expression in populations identified in panel A from lung samples taken from four independent donors, corrected for nonspecific staining using unpermeabilized controls for each sample, and error bars represent the SEM. (C) Median IRF5 expression in myeloid versus lymphoid cell subsets, and error bars represent the SEM.
FIG 4
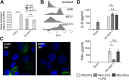
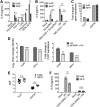
_IRF5_−/− iPSCs, IRF5Comp iPSCs, and Kolf2 iPSCs can be differentiated into iPS-DCs which lack or express IRF5. CRISPR-Cas9 was used to generate biallelic mutations in IRF5 in the Kolf2 background. IRF5Comp iPSCs were generated using TALEN-mediated integration of IRF5 into the IRF5−/− background. (A) Relative expression of IRF5 in iPSCs and iPS-DCs relative to GAPDH. Data are shown as four technical replicates per assay, with assays repeated three times from independent iPS-DC batches. (B) Flow cytometry showing IRF5 expression in iPS-DCs generated from IRF5−/−, IRF5Comp, and Kolf2 iPSCs. (C) Immunostaining for IRF5 in A/X-31 influenza (IAV)-infected Kolf2 and IRF5−/− iPS-DCs (DAPI, blue; IRF5, green). (D) IL-6 and TNF-α production 24 h p.i. by IAV-challenged Kolf2 iPS-DCs and monocyte-derived DCs generated from human peripheral blood, either with or without 48 h LPS maturation, were assayed by ELISA. Data represented show the mean ± SEM from three independent Kolf2 differentiations for iPS-DCs, and from three independent healthy donors for monocyte-derived DCs.
FIG 5
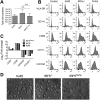
_IRF5_−/− iPSCs, IRF5Comp iPSCs, and Kolf2 iPSCs can be differentiated into iPS-DCs that display similar morphologies. _IRF5_−/− iPSCs, IRF5Comp iPSCs, and Kolf2 iPSCs were differentiated into dendritic cells using defined concentrations of growth factors to generate embryoid bodies (EBs) and GM-CSF and IL-4 to generate immature DCs from these EBs. (A) Total cell numbers of DC precursors harvested from DC differentiation plates. Data shown are from 8 independent differentiations per iPSC line. (B) Surface expression of DC markers was examined via flow cytometry in Kolf2, IRF5−/−, and IRF5Comp iPS-DCs. Representative plots are presented from one experiment, with experiments performed at least three times. (C) Gene expression of DC markers CD83 and CD86 and iPSC markers NANOG and POU5F1 by iPS-DCs relative to GAPDH was quantified using TaqMan gene expression assays. The data shown represent four technical replicates per assay, with assays repeated at least twice from independent iPS-DC batches. (D) Morphologies of iPS-DCs generated from Kolf2, IRF5Comp, and IRF5−/− iPSCs.
FIG 6
IRF5 enhances IAV-induced inflammatory cytokine production in iPS-DCs and iPSDMs. (A) IL-6 and TNF-α were measured by ELISA in supernatants harvested from iPS-DCs and iPSDMs generated from an iPSC line with a biallelic mutation in IRF5, compared to the parent line Kolf2, and a line with a functional IRF5 gene was reintroduced into the AAVS1 integration site by TALEN engineering after infection with IAV at an MOI of 1. Supernatants were harvested at 24 h for assays; data shown represent the mean ± SEM for triplicate wells from at least 3 independent experiments. (B) IRF5−/−, IRF5Comp, and Kolf2 iPS-DCs were infected with IAV at an MOI of 1 and then stained for IAV NP 24 h postinfection and analyzed via flow cytometry. SSC-H, side scatter height. (C) Percentage of positive NP iPS-DCs 24 h postinfection with IAV, with data presented showing the mean ± SEM from three independent experiments. (D) Expression of DC maturation surface markers for iPS-DCs generated from IRF5−/−, Kolf2, or IRF5Comp hIPSCs 24 h postinfection with A/X-31 influenza (IAV) at an MOI of 1, as measured by flow cytometry, with data presented showing the mean ± SEM from three independent experiments.
FIG 7
Type I IFN signaling enhances IL-6 and TNF-α production by iPS-DCs. A total of 2 × 104 iPS-DCs were challenged as stated below for each assay, and supernatants were harvested after 24 h, unless otherwise stated. A/X-31 influenza (IAV) was used at an MOI of 1. (A) Cells were preincubated for 1 h with anti-IFNAR1 antibody or left untreated prior to viral infection. Data shown represent the mean ±SEM for triplicate wells from at least 3 experiments. Supernatants were harvested and assayed for IL-6 and TNF-α by ELISA. (B) Supernatants from mock or IAV-infected Kolf2 or IRF5−/− iPS-DCs were harvested at 24 h and assayed for IFN-α and IFN-β by ELISA. Data shown represent 2 separate experiments.
FIG 8
IRF5 acts downstream of TLR7 and RIG-I to drive inflammatory cytokine responses in iPS-DCs. A total of 2 × 104 iPS-DCs were challenged as stated below for each condition in each assay, and supernatants were harvested after 24 h. A/X-31 influenza virus (IAV) was used at an MOI of 1. For blocking assays, cells were either preincubated for 1 h with inhibitor (IMD 0354, IKKβ inhibitor), or inhibitor was added directly with viral inoculum (ODN 20958, TLR7 inhibitor). Data shown represent the mean ± SEM of the results for triplicate wells from at least 3 experiments, unless otherwise stated. (A) IL-6 production by Kolf2 and IRF5−/− iPS-DCs in response to stimulation with various TLR ligands (TLR2, Pam3CSK4, 300 ng/ml; TLR3, poly(I·C), 50 μg/ml; TLR4, LPS, 50 μg/ml; TLR7, imiquimod, 50 μg/ml; TLR9, ODN 2216, 3 μg/ml) was measured by ELISA. Data shown represent four wells per condition for one iPS-DC batch per line, with assays replicated in two independent experiments. (B) IL-6 response as measured by ELISA in Kolf2 and IRF5−/− iPS-DCs to RIG-I ligand 3p-hpRNA with or without IKKβ inhibitor IMD 0354 and to IAV with or without IMD 0354. (C) Fold change in mRNA levels for TLR7 and DDX58, measured by RT-qPCR using GAPDH as an endogenous control. (D) DDX58 and TLR7 mRNA levels in iPS-DCs after IAV infection with or without blocking of type I IFN signaling using anti-IFNAR1. Data shown represent four technical replicates per assay, with assays repeated at least twice from independent iPS-DC batches. (E) Relative mRNA levels of TLR7 in iPS-DCs generated from IRF5−/− iPSCs or parent Kolf2 iPSCs, measured using RT-qPCR. (F) IL-6 response as measured by ELISA in Kolf2 and IRF5−/− iPS-DCs to A/X-31 influenza virus with or without TLR7 inhibitor ODN 20958.
Similar articles
- Identification of lncRNA-155 encoded by MIR155HG as a novel regulator of innate immunity against influenza A virus infection.
Maarouf M, Chen B, Chen Y, Wang X, Rai KR, Zhao Z, Liu S, Li Y, Xiao M, Chen JL. Maarouf M, et al. Cell Microbiol. 2019 Aug;21(8):e13036. doi: 10.1111/cmi.13036. Epub 2019 May 29. Cell Microbiol. 2019. PMID: 31045320 - Inducible Guanylate-Binding Protein 7 Facilitates Influenza A Virus Replication by Suppressing Innate Immunity via NF-κB and JAK-STAT Signaling Pathways.
Feng M, Zhang Q, Wu W, Chen L, Gu S, Ye Y, Zhong Y, Huang Q, Liu S. Feng M, et al. J Virol. 2021 Feb 24;95(6):e02038-20. doi: 10.1128/JVI.02038-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33408175 Free PMC article. - Type I Interferon Induced Epigenetic Regulation of Macrophages Suppresses Innate and Adaptive Immunity in Acute Respiratory Viral Infection.
Kroetz DN, Allen RM, Schaller MA, Cavallaro C, Ito T, Kunkel SL. Kroetz DN, et al. PLoS Pathog. 2015 Dec 28;11(12):e1005338. doi: 10.1371/journal.ppat.1005338. eCollection 2015 Dec. PLoS Pathog. 2015. PMID: 26709698 Free PMC article. - Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.
Nogales A, Martinez-Sobrido L, Topham DJ, DeDiego ML. Nogales A, et al. Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review. - Innate Immune Sensing of Influenza A Virus.
Malik G, Zhou Y. Malik G, et al. Viruses. 2020 Jul 14;12(7):755. doi: 10.3390/v12070755. Viruses. 2020. PMID: 32674269 Free PMC article. Review.
Cited by
- Broad Impact of Exchange Protein Directly Activated by cAMP 2 (EPAC2) on Respiratory Viral Infections.
Choi EJ, Wu W, Cong X, Zhang K, Luo J, Ye S, Wang P, Suresh A, Ullah UM, Zhou J, Bao X. Choi EJ, et al. Viruses. 2021 Jun 21;13(6):1179. doi: 10.3390/v13061179. Viruses. 2021. PMID: 34205489 Free PMC article. - Monocytopenia, monocyte morphological anomalies and hyperinflammation characterise severe COVID-19 in type 2 diabetes.
Alzaid F, Julla JB, Diedisheim M, Potier C, Potier L, Velho G, Gaborit B, Manivet P, Germain S, Vidal-Trecan T, Roussel R, Riveline JP, Dalmas E, Venteclef N, Gautier JF. Alzaid F, et al. EMBO Mol Med. 2020 Oct 7;12(10):e13038. doi: 10.15252/emmm.202013038. Epub 2020 Sep 11. EMBO Mol Med. 2020. PMID: 32816392 Free PMC article. - Human pluripotent stem cells for the modelling and treatment of respiratory diseases.
Goldsteen PA, Yoseif C, Dolga AM, Gosens R. Goldsteen PA, et al. Eur Respir Rev. 2021 Aug 3;30(161):210042. doi: 10.1183/16000617.0042-2021. Print 2021 Sep 30. Eur Respir Rev. 2021. PMID: 34348980 Free PMC article. Review. - IFITM3 restricts virus-induced inflammatory cytokine production by limiting Nogo-B mediated TLR responses.
Clement M, Forbester JL, Marsden M, Sabberwal P, Sommerville MS, Wellington D, Dimonte S, Clare S, Harcourt K, Yin Z, Nobre L, Antrobus R, Jin B, Chen M, Makvandi-Nejad S, Lindborg JA, Strittmatter SM, Weekes MP, Stanton RJ, Dong T, Humphreys IR. Clement M, et al. Nat Commun. 2022 Sep 8;13(1):5294. doi: 10.1038/s41467-022-32587-4. Nat Commun. 2022. PMID: 36075894 Free PMC article. - RNA regulatory mechanisms that control antiviral innate immunity.
Gokhale NS, Smith JR, Van Gelder RD, Savan R. Gokhale NS, et al. Immunol Rev. 2021 Nov;304(1):77-96. doi: 10.1111/imr.13019. Epub 2021 Aug 17. Immunol Rev. 2021. PMID: 34405416 Free PMC article. Review.
References
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJD, Chau TNB, Hoang DM, Van Vinh Chau N, Khanh TH, Dong VC, Qui PT, Van Cam B, Ha DQ, Guan Y, Peiris JSM, Chinh NT, Hien TT, Farrar J. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12:1203–1207. doi:10.1038/nm1477. - DOI - PMC - PubMed
- GeurtsvanKessel CH, Willart MAM, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus A, Rimmelzwaan GF, Lambrecht BN. 2008. Clearance of influenza virus from the lung depends on migratory langerin+ CD11b− but not plasmacytoid dendritic cells. J Exp Med 205:1621–1634. doi:10.1084/jem.20071365. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- MR/L018942/1/MRC_/Medical Research Council/United Kingdom
- WT098051/WT_/Wellcome Trust/United Kingdom
- 207503/Z/17/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials