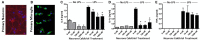Neuron-Specific Vitamin D Signaling Attenuates Microglia Activation and CNS Autoimmunity - PubMed (original) (raw)
Neuron-Specific Vitamin D Signaling Attenuates Microglia Activation and CNS Autoimmunity
Priscilla W Lee et al. Front Neurol. 2020.
Abstract
Low vitamin D during childhood is associated with an increased risk of developing multiple sclerosis (MS) as an adult. Given that vitamin D has anti-inflammatory properties, it has been postulated that the relationship between MS and low vitamin D is due to immune dysregulation. Since the vitamin D receptor (VDR) is expressed in many cell types, this study investigated an alternative hypothesis-neuron-specific VDR signaling induces anti-inflammatory molecules that protect the central nervous system from autoimmunity. Using media from neurons treated with calcitriol, the active form of vitamin D3, LPS-activated microglia had a reduction in pro-inflammatory molecules, and a reciprocal induction of anti-inflammatory molecules. Since IL-34 is critical to the homeostasis of microglia, and was previously shown to be induced in endothelial cells by vitamin D, we investigated IL-34 as the potential anti-inflammatory molecule induced in neurons by vitamin D. Treatment of LPS-activated microglia with IL-34 reduced pro-inflammatory cytokine production and enhanced the expression of anti-inflammatory transcripts. However, neutralizing IL-34 in vitamin D neuronal conditioned media only impacted IL-6 and not the broader anti-inflammatory phenotype of microglia. To mimic low vitamin D in children, we used a neuron-specific inducible mouse model in which VDR was partially deleted in juvenile mice. Partial deletion of VDR in neurons during early life resulted in exacerbated CNS autoimmunity in adult mice. Overall, the study illustrated that vitamin D signaling in neurons promotes an anti-inflammatory state in microglia, and low vitamin D in early life may enhance CNS autoimmunity.
Keywords: experimental autoimmune encephalomyelitis; microglia; multiple sclerosis; neurons; vitamin D.
Copyright © 2020 Lee, Selhorst, Lampe, Liu, Yang and Lovett-Racke.
Figures
Figure 1
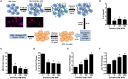
Vitamin D signaling in neurons reduces microglial activation. (A) N2a cells were differentiated into neuronal-like cells using retinoic acid, treated with calcitriol (0–1,000 nM), and the media collected (NCM). Micrographs illustrate the N2a cells before and after 7 days with retinoic acid stained for tuj1 [neuron-specific class III beta-tubulin (Red—β-tubulin; Blue—DAPI)]. The BV-2 microglia cell line was placed in culture, treated with NCM for 24 h, washed, and activated with LPS. After 8 h, IL-6 was measured in the BV-2 supernatant (B), and transcripts for MCHII (C), Nos2 (D), Hmox1 (E), and Arg1 (F) were measured by real-time PCR in the microglia. *p < 0.05.
Figure 2
Vitamin D signaling in primary neurons reduces pro-inflammatory cytokine production by microglia. (A) Primary neurons were isolated from the hippocampus of post-natal day 1 mice. Red—β-tubulin; Blue—DAPI. (B) Primary microglia stained with Iba1 (green) and DAPI (blue). The primary neurons were cultured with calcitriol and the media was collected and transferred to the primary microglia. After 24 h, the microglia were washed, activated with LPS, supernatants collected, and IL-6 (C), IL-1β (D), and TNFα (E) were measured in the supernatants by ELISA. *p < 0.05.
Figure 3
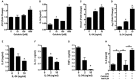
Vitamin D induces IL-34 in neurons, and IL-34 induces an anti-inflammatory phenotype in microglia. (A) IL34 transcripts were measured by real-time PCR in primary neurons treated with calcitriol. (B) IL-34 was measured by ELISA in the supernatants of primary neurons treated with calcitriol. Hmox1 (C) and Arg1 (D) transcripts were measured by real-time PCR in primary microglia treated with IL-34 and activated with LPS. IL-6 (E), IL-1β (F), and TNFα (G) were measured in the supernatants of primary microglia treated with IL-34 and activated with LPS. (H) NCM from calcitriol-treated primary neurons was treated with anti-IL34, transferred to primary microglia, and IL-6 was measured by ELISA. *p < 0.05.
Figure 4
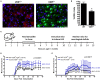
Reduced VDR signaling in neurons during early life enhances CNS autoimmunity. VDRf/+ mice were crossed with SLICK-H [Tg(Thy1-cre/ERT2,-EYFP)HGfng/PyngJ] to generate SLICK/VDRf/+ mice. Experiments used littermates and all mice were fed tamoxifen chow from 3 to 5 weeks of age and then returned to standard chow. (A) The brains and spinal cords were analyzed for VDR expression—red = VDR, green = Cre-EYFP, blue = DAPI. (B) Quantification of VDR+ cells in the brain and spinal cord, normalized to WT mice. N = 7 per group, *p < 0.05. (C,D) Mice were fed tamoxifen chow at 3–5 weeks and immunized at 8–10 weeks with 50 μg MOG35-55 and 50 μg PLP139-151 homogenized in CFA containing 2 mg/ml Mycobacterium tuberculosis, followed by an i.p. injection with pertussis toxin (100 ng/mouse). Controls = 10 and SLICK/VDRf/+ = 6 mice. p < 0.0001 for EAE scores (Mann-Whitney). (E) Mice were fed tamoxifen chow at 3–5 weeks and immunized at 8–10 weeks with 12 μg MOG35-55 and 12 μg PLP139-151 homogenized in CFA containing 1 mg/ml Mycobacterium tuberculosis, followed by an i.p. injection with pertussis toxin (25 ng/mouse). Controls = 6 and SLICK/VDRf/+ = 4 mice. p < 0.0017 for EAE scores (Mann-Whitney). Data is representative of ≥3 experiments.
Similar articles
- Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism.
Boontanrart M, Hall SD, Spanier JA, Hayes CE, Olson JK. Boontanrart M, et al. J Neuroimmunol. 2016 Mar 15;292:126-36. doi: 10.1016/j.jneuroim.2016.01.015. Epub 2016 Jan 27. J Neuroimmunol. 2016. PMID: 26943970 - Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue.
Smolders J, Schuurman KG, van Strien ME, Melief J, Hendrickx D, Hol EM, van Eden C, Luchetti S, Huitinga I. Smolders J, et al. J Neuropathol Exp Neurol. 2013 Feb;72(2):91-105. doi: 10.1097/NEN.0b013e31827f4fcc. J Neuropathol Exp Neurol. 2013. PMID: 23334593 - Neuronal injury in chronic CNS inflammation.
Zindler E, Zipp F. Zindler E, et al. Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review. - Drug-induced microglial phagocytosis in multiple sclerosis and experimental autoimmune encephalomyelitis and the underlying mechanisms.
Ju WY, Wang Q, Song LJ, Ding ZB, Li XH, Kumar G, Yan Y, Ma CG. Ju WY, et al. Mol Biol Rep. 2023 Jan;50(1):749-759. doi: 10.1007/s11033-022-07968-z. Epub 2022 Oct 29. Mol Biol Rep. 2023. PMID: 36309614 Review.
Cited by
- Brain nuclear receptors and cardiovascular function.
Wang M, Yang Y, Xu Y. Wang M, et al. Cell Biosci. 2023 Jan 20;13(1):14. doi: 10.1186/s13578-023-00962-3. Cell Biosci. 2023. PMID: 36670468 Free PMC article. Review. - Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases.
Holick MF, Mazzei L, García Menéndez S, Martín Giménez VM, Al Anouti F, Manucha W. Holick MF, et al. Nutrients. 2023 Feb 2;15(3):767. doi: 10.3390/nu15030767. Nutrients. 2023. PMID: 36771473 Free PMC article. Review. - Vitamin D as a Risk Factor for Multiple Sclerosis: Immunoregulatory or Neuroprotective?
Gombash SE, Lee PW, Sawdai E, Lovett-Racke AE. Gombash SE, et al. Front Neurol. 2022 May 16;13:796933. doi: 10.3389/fneur.2022.796933. eCollection 2022. Front Neurol. 2022. PMID: 35651353 Free PMC article. Review. - Microglia and Brain Disorders: The Role of Vitamin D and Its Receptor.
Mirarchi A, Albi E, Beccari T, Arcuri C. Mirarchi A, et al. Int J Mol Sci. 2023 Jul 25;24(15):11892. doi: 10.3390/ijms241511892. Int J Mol Sci. 2023. PMID: 37569267 Free PMC article. Review. - Obesity Control and Supplementary Nutraceuticals as Cofactors of Brain Plasticity in Multiple Sclerosis Populations.
Ciumărnean L, Sârb OF, Drăghici NC, Sălăgean O, Milaciu MV, Orășan OH, Vlad CV, Vlad IM, Alexescu T, Para I, Țărmure SF, Hirișcău EI, Dogaru GB. Ciumărnean L, et al. Int J Mol Sci. 2024 Oct 10;25(20):10909. doi: 10.3390/ijms252010909. Int J Mol Sci. 2024. PMID: 39456690 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases