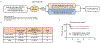Epigenetic therapy inhibits metastases by disrupting premetastatic niches - PubMed (original) (raw)
. 2020 Mar;579(7798):284-290.
doi: 10.1038/s41586-020-2054-x. Epub 2020 Feb 26.
Zhihao Lu # 1 2 3, Shuang Li # 2, Michael J Topper 3, Yong Tao 3, Hao Zhang 4, Xi Jiao 2, Wenbing Xie 3, Xiangqian Kong 3, Michelle Vaz 3, Huili Li 3, Yi Cai 3, Limin Xia 3 5, Peng Huang 3, Kristen Rodgers 1, Beverly Lee 1, Joanne B Riemer 3, Chi-Ping Day 6, Ray-Whay Chiu Yen 3, Ying Cui 3, Yujiao Wang 2, Yanni Wang 2, Weiqiang Zhang 1 7, Hariharan Easwaran 3, Alicia Hulbert 1 8, KiBem Kim 3, Rosalyn A Juergens 9, Stephen C Yang 1, Richard J Battafarano 1, Errol L Bush 1, Stephen R Broderick 1, Stephen M Cattaneo 10, Julie R Brahmer 3, Charles M Rudin 11, John Wrangle 12, Yuping Mei 1 3, Young J Kim 13, Bin Zhang 14 15, Ken Kang-Hsin Wang 14, Patrick M Forde 3 16, Joseph B Margolick 4, Barry D Nelkin 3, Cynthia A Zahnow 3, Drew M Pardoll 3 16, Franck Housseau 17 18, Stephen B Baylin 19, Lin Shen 20, Malcolm V Brock 21 22
Affiliations
- PMID: 32103175
- PMCID: PMC8765085
- DOI: 10.1038/s41586-020-2054-x
Epigenetic therapy inhibits metastases by disrupting premetastatic niches
Zhihao Lu et al. Nature. 2020 Mar.
Abstract
Cancer recurrence after surgery remains an unresolved clinical problem1-3. Myeloid cells derived from bone marrow contribute to the formation of the premetastatic microenvironment, which is required for disseminating tumour cells to engraft distant sites4-6. There are currently no effective interventions that prevent the formation of the premetastatic microenvironment6,7. Here we show that, after surgical removal of primary lung, breast and oesophageal cancers, low-dose adjuvant epigenetic therapy disrupts the premetastatic microenvironment and inhibits both the formation and growth of lung metastases through its selective effect on myeloid-derived suppressor cells (MDSCs). In mouse models of pulmonary metastases, MDSCs are key factors in the formation of the premetastatic microenvironment after resection of primary tumours. Adjuvant epigenetic therapy that uses low-dose DNA methyltransferase and histone deacetylase inhibitors, 5-azacytidine and entinostat, disrupts the premetastatic niche by inhibiting the trafficking of MDSCs through the downregulation of CCR2 and CXCR2, and by promoting MDSC differentiation into a more-interstitial macrophage-like phenotype. A decreased accumulation of MDSCs in the premetastatic lung produces longer periods of disease-free survival and increased overall survival, compared with chemotherapy. Our data demonstrate that, even after removal of the primary tumour, MDSCs contribute to the development of premetastatic niches and settlement of residual tumour cells. A combination of low-dose adjuvant epigenetic modifiers that disrupts this premetastatic microenvironment and inhibits metastases may permit an adjuvant approach to cancer therapy.
Figures
Extended Data Figure 1.. Efficacy of low dose adjuvant epigenetic therapy on cancer recurrence in stage I (T1– 2aN0) NSCLC patients in a phase II clinical trial.
a, Schema for a randomized phase II clinical trial of adjuvant epigenetic therapy in stage I (T1–2aN0) NSCLC patients (
NCT01207726
). b, Postsurgical recurrence rates in the observation and adjuvant epigenetic therapy groups. c, Kaplan-Meier curves for disease-free survival in the observation and adjuvant epigenetic therapy groups. _P_=0.50 by two-sided log-rank test.
Extended Data Figure 2.. Schema outlining the establishment and characteristics of the murine pulmonary metastasis models.
a, Schema for establishing the highly aggressive HNM007 pulmonary metastasis model. Pulmonary metastases were harvested and serially subcutaneously implanted in the right flanks of mice for 10 passages. b, Schema for establishing the 4T1 pulmonary metastasis model. c, Characteristics of murine cell line (non-selective) and spontaneous murine tissue pulmonary metastasis models (metastases harvested selectively from serial pulmonary metastases to produce a solely pulmonary metastatic phenotype). d, Longitudinal gross pathological photographs of bilateral pulmonary metastases during the natural history of the LLC pulmonary metastasis model in C57BL/6 mice from day 0 to day 15 after surgery. N1, N2, and N3 depict the experiment performed in triplicate. Two mice were sacrificed at each time point from Day 0 to Day 15 (n=36) of which 18 mice are shown here as representative photomicrographs. e, H&E staining of pulmonary metastases in LLC, HNM007 and 4T1 pulmonary metastasis mice. The histology of LLC (day 9), HNM007 (day 12) and 4T1 (day 12) pulmonary metastases were confirmed by a pathologist. Scale bar, 100μm. Representative data were repeated at least three times with similar results.
Extended Data Figure 3.. CD11b+Gr-1+ cells persist as the predominant immune cells even post-resection in the lung premetastatic microenvironment as functional MDSCs.
a, In LLC metastasis mice, lung CD11b+Ly6ChighLy6G− and CD11b+Ly6ClowLy6G+ cells harvested at 72 hours post-resection both have suppressive activity in vitro against CD8a T-cells. Freshly isolated CD11b+Ly6ChighLy6G− or CD11b+Ly6ClowLy6G+ cells from both lungs at day 3 post-resection were co-cultured with CD8a T-cells for 72 hours at different ratios (0:1, 1:1, 2:1, 4:1 and 1:0). T-cell proliferation and IFN-γ concentrations in the supernatant were measured by FACS (left panels) and ELISA (right panels), respectively (n=3 biological replicates). Representative data were repeated at least three times with similar results. Two-sample two-sided t-test was used in the comparison with mock (CD8a T-cells alone). b, Immune cell profiles of liver in LLC metastasis mice. Single cell suspensions from the entire liver were analyzed by FACS (n=3 mice per timepoint) at different timepoints after surgery. NC: negative control, normal liver from C57BL/6 mice. c, Immune cell profiles of both lungs in HNM007 metastasis mice at different timepoints after surgery. Single cell suspensions from both lungs were analyzed by FACS (n=3 mice per timepoint). NC: negative control, normal lungs from C57BL/6 mice. b and c, two-sample two-sided t-test was used in comparison with NC. All bars show mean ± s.e.m. *P < 0.05, **P < 0.01, ***P< 0.001.
Extended Data Figure 4.. Consideration of combined adjuvant epigenetic treatment dosing based on its effect on murine models.
a, The upper panels show the effect of different dosages of epigenetic modifiers on the viability of LLC1, HNM007 and 4T1 cells in vitro (72 hours, Cell Counting Kit-8). Graphs show the mean of 3 independent experiments, two-sample two-sided t-tests with mock; The lower panels show the effect of low dose Aza (100nM) plus entinostat (50nM) on the proliferation of LLC1, HNM007 and 4T1 cells in vitro. A total of 1 × 105 viable cells were plated per well. Cells were collected at 24, 48 and 72 hours and counted using a cell counter (Bio-Rad) after Trypan blue exclusion. Graphs show mean of 3 independent experiments, significance at 72h was determined by one-way ANOVA followed by Tukey’s test for multiple comparisons. b, The effect of low dose Aza (100nM) plus entinostat (50nM) on the viability of BM-MDSCs (day 3) from LLC (upper) and HNM007 (lower) metastasis mice in vitro (Cell Counting Kit-8). Graphs show the mean of 3 independent experiments. c, The effect of low dose Aza (100nM) plus entinostat (50nM) on the apoptosis of BM-MDSCs (day 3) from LLC and HNM007 metastasis mice in vitro. Cell apoptosis was measured by FACS at 48 hours. The lower right quadrant (Annexin-V+/7-AAD−) and upper right quadrant (Annexin-V+/7-AAD +) represent early and late apoptotic cells, respectively. Graphs show the percentage of total apoptosis (early and late apoptosis) in mock and treatment groups (n=3 biological replicates). d, Tumour growth and body weight of LLC tissue-bearing NSG mice treated with different doses of entinostat plus Aza. Significance at day 12 (upper panels) and day 14 (lower panels) was determined by one-way ANOVA followed by Tukey’s test for multiple comparisons. e, Summary Table of tumour growth, body weight, and treatment-related death of LLC tissue-bearing NSG mice. Regimens in red indicate dosages with no effect on tumour growth, weight loss or treatment-related death. f, Tumour growth and body weight of HNM007 tissue-bearing NSG mice treated with Aza 0.5mg/kg/day plus entinostat 5mg/kg/day or vehicle. Significance at day 14 was determined by two-sample two-sided t-test. The effect of LD-AET on the proliferation (g) and apoptosis (h) of donor-derived CD45.1+ BM-MDSCs in CD45.2 LLC metastasis mice. Proliferation and apoptosis of immature (MHC-II−) and mature (MHC-II+) CD45.1+ cells were measured by FACS at 36 hours after transfusion (day 2). Graphs (g) show the percentage of Ki67+ cells (n=3 mice per group). Graphs (h) show the percentage of total apoptosis (early and late apoptosis) in mock and LD-AET groups (n=3 mice per group). b, c, g, and h, two-sample two-sided t-test. All bars show mean ± s.e.m. *P < 0.05.
Extended Data Figure 5.. Low dose adjuvant epigenetic therapy disrupts the lung premetastatic microenvironment mainly by affecting MDSCs
a, The effect of LD-AET (Aza 0.5mg/kg/day plus entinostat 2.5mg/kg/day) on lung MDSCs at day 3 post-resection in 4T1 metastasis mice (n=3 per group). Immunofluorescence staining of CD4+ and CD8+ T-cells (b), or Gr-1+ cells (c) from the lung premetastatic microenvironment (day3) in LLC metastasis mice with or without LD-AET. Negative control, normal lungs from tumour-free C57BL/6 mice. Immunofluorescence staining was performed using CD4 (green) and CD8 (red) antibodies, or Gr-1 (red) antibodies. Merged images contain DAPI staining for cell nuclei (blue). Original magnification 20×. Representative data were repeated at least three times with similar results. The mRNA (d) and protein (e) levels of representative molecular factors known to promote premetastatic microenvironment formation from both lungs of normal mice, mock LLC metastasis mice (day 3), and LD-AET treated LLC metastasis mice (day 3) were measured by quantitative PCR and western blot. Two-sample two-sided t-test for quantitative PCR experiments (n=3 biological replicates). For gel source data, see Supplementary Fig.1. All the experiments were performed in triplicate and similar results were obtained. f, Graphs (upper panels) showing the percentages of donor-derived cell subsets (CD45.1+ MDSC cells) in the lungs of LLC metastasis mice or sham surgery mice (tumour-naive recipient mice) thirty-six hours after surgery; Graphs (lower panels) showing the percentages of donor-derived cell subsets (CD45.1+ MDSC cells) in the lungs of LD-AET or vehicle treated sham surgery mice (tumour-naïve recipient mice) thirty-six hours after surgery. Purified 5× 106 bone marrow MDSCs from LLC tumour-bearing CD45.1+ mice (day 0) were adoptively transferred into CD45.2+ recipient mice in the sham surgery tumour-naive model or LLC metastatic model (n=3 mice per group). a, f, two-sample two-sided t-test. All bars show mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
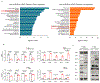
Extended Data Figure 6.. Low dose adjuvant epigenetic therapy induces substantial changes in immune cell chemotaxis and migration in MDSCs in LLC pulmonary metastasis mice.
a, Schema (left) showing the effect of LD-AET on M-MDSCs or PMN-MDSCs transferred from CD45.1 to CD45.2 C57BL/6 mice in LLC metastasis model. CD45.1+ cells (transferred PMN-MDSCs) were identified in the lungs of the recipient mice and analyzed by flow cytometry (right, n=3 mice per group). b, Schema (left) showing trafficking ability of adoptively transferred M-MDSCs or PMN-MDSCs from LD-AET treated or untreated CD45.1 mice in LLC metastasis model. CD45.1+cells (transferred PMN-MDSCs) were identified in the lungs of the recipient CD45.2 mice and analyzed by flow cytometry at 18 hours after transfer (right, n=3 mice per group). c, Top ten upregulated gene sets from GSEA of lung M-MDSCs after 72 hours of treatment with LD-AET (left panel). Representative upregulated GSEA plots of immune cell chemotaxis and migration (right panels). NES: normalized enrichment score. FDR: false discovery rate. Colour gradation is representative of log2 fold change over mock (n=3 biologically replicates). Gene set enrichment P values, NES values and FDR values reported are calculated with 1,000 permutations in the GSEA software. FDR q-value < 0.25 was deemed significant. d, DAVID analyses of significantly downregulated genes using the KEGG gene ontology in BM-MDSCs from LLC metastasis mice treated or untreated with LD-AET. Top 20 downregulated pathways are presented (n=3 biologically replicates). Hypergeometric test (FDR adjusted p-value<0.05). e, LD-AET significantly decrease nuclear activation of p52 and Rel-B [OD450nm (Mock versus LD-AET): p52: 0.95±0.035 versus 0.721±0.011, _P_=0.0034; Rel-B: 0.251±0.012 versus 0.1±0.003, _P_=0.0002], but not p50 and p65 in BM-M-MDSCs from LLC metastasis mice in vivo. Nuclear lysates were incubated with oligonucleotides containing the NF-κB-binding consensus sequence, and specific antibodies were used to detect the different subunits within the bound complexes (n=3 biologically replicates). f, FACS shows the effect of 30mg/kg/d and 75mg/kg/d of BMS-345541 (a highly selective IKB kinase inhibitor) on CCR2 expression in BM-M-MDSCs from LLC metastasis mice on day 3 after surgery. The experiments were performed in triplicate, and similar results were obtained. g, CXCR1 and CXCR2 expression of PMN-MDSCs harvested on day 3 from the bone marrow and or lung detected by quantitative-PCR (upper) and FACS (lower) in LLC metastasis mice treated with vehicle or with 72 hours of LD-AET (n=3 biological replicates). h, Transwell migration assay of sorted BM-PMN-MDSCs from LD-AET (72 hours) or vehicle treated LLC metastasis mice induced by CXCL1 (20ng/ml and 50ng/ml) for 120 minutes (n=3 biological replicates). a-b, e, g-h, two-sample two-sided t-test. All bars show mean ± s.e.m.
Extended Data Figure 7.. Low dose adjuvant epigenetic therapy promotes the differentiation of M-MDSCs towards macrophages in the LLC pulmonary metastasis model
a, DAVID analyses of the significantly downregulated and upregulated genes using the KEGG gene ontology in LLC metastasis mice treated or untreated with LD-AET (n=3 biological replicates). Hypergeometric test (FDR adjusted p-value<0.05). The mRNA (b) and protein (c) levels of representative transcription factors were measured by quantitative PCR and western blot, respectively. In vitro, splenic M-MDSCs from LLC metastasis mice were cultured for 3 days with tumour conditioned medium (TCM). In vivo, M-MDSCs from both lungs of mock (day 3) and LD-AET treated LLC metastasis mice (day 3) were sorted for analysis. For gel source data, see Supplementary Fig. 1. Representative data were repeated at least three times with similar results. b, two-sample two-sided t-test, n=3 biological replicates. All bars show mean ± s.e.m.
Extended Data Figure 8.. Low dose adjuvant epigenetic therapy promotes the differentiation of M-MDSCs towards an interstitial macrophage-like population in the lung premetastatic microenvironment
a, Gating strategy used to identify and analyze lung interstitial macrophages in the lung premetastatic microenvironment by FACS. b, The effect of LD-AET on lung interstitial macrophages from the LLC metastasis mice. The percentage and cell counts of interstitial macrophages from both lungs in mock and LD-AET mice were analyzed by FACS at day 3 after surgery (n=3 per group). Two-sample two-sided t-test. All bars show mean ± s.e.m. c, Gating strategy used to identify and analyze CD45.1+ lung interstitial macrophages from the lungs of recipient CD45.2 mice after the transfusion of CD45.1+ M-MDSCs. d, Kaplan-Meier curves showing the disease-free and overall survival of the LLC CCR2 KO metastasis mice after transfusion of CCR2 WT M-MDSCs (5×106), LD-AET treated (in vivo) CCR2 WT M-MDSCs (5× 106), or vehicle at day1 and day4, respectively. e, Kaplan-Meier curves showing the disease-free and overall survival of the LLC CCR2 KO metastasis mice after transfusion of CCR2 WT PMN-MDSCs (5× 106), LD-AET treated (in vivo) CCR2 WT PMN-MDSCs (5× 106), or vehicle at day1 and day4, respectively. d-e, two-sided log-rank test.
Extended Data Figure 9.. Low dose adjuvant epigenetic therapy inhibits pulmonary metastases and prolongs overall survival in murine models
a, Representative photographs showing lungs treated with vehicle or LD-AET in LLC (day6) and HNM007 (day10) metastasis mice. The red arrows indicate the metastases. b, Representative cone beam computed tomography (CBCT) images of lung metastases on day 6 post-resection in LLC metastasis mice treated with vehicle or LD-AET. The red arrows indicate the metastases. c, Representative H&E-stained images of lung sections from HNM007 (upper panels) and 4T1 (lower panels) metastasis mice treated with LD-AET or vehicle at different time points after surgery. Scale bar, 2 mm. Graph shows area and numbers of metastatic nodules. At each time point, 3 mice were sacrificed for analysis. For each sample, sections from 3 levels were analyzed. Two-sample two-sided t-test. d, Kaplan-Meier curves showing the disease-free and overall survival of HNM007 and 4T1 metastasis mice treated with LD-AET (for 4T1 metastasis model, Aza 0.5mg/kg/day plus entinostat 2.5mg/kg/day) or vehicle after surgery. e, FACS showing the effect of T-cell depleting antibodies on CD4+ and CD8+ T-cells in the peripheral blood of the LLC metastasis mice. n=3 mice per group. Two-sample two-sided t-test. f, Kaplan-Meier curves showing the disease-free and overall survival of LLC metastasis mice treated with vehicle, CCR2 antagonist (RS102895, Sigma), LD-AET, and RS102895 plus LD-AET after surgery. g, Kaplan-Meier curves showing the disease-free survival and overall survival of HNM007 metastases mice treated with vehicle, CCR2 antagonist (RS504393, Sigma), LD-AET and RS504393 in combination with LD-AET. d, f-g, two-sided log-rank test. Representative data in a-b were repeated at least three times with similar results. All bars show mean ± s.e.m. *P< 0.05, **P <0.01, ***P< 0.001.
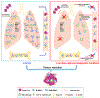
Extended Data Figure 10.. Representation of the effect of low dose adjuvant epigenetic modifiers on lung metastases in the tumour pulmonary metastasis models.
Graphic model showing the inhibition of pulmonary metastases by LD-AET via its effect on MDSCs. Firstly, LD-AET can inhibit the trafficking of M-MDSCs and PMN-MDSCs from the bone marrow to the premetastatic microenvironment by downregulating the expression of CCR2 and CXCR2, respectively. Secondly, even if MDSCs migrate to the lung, LD-AET can skew the differentiation of M-MDSCs towards an interstitial macrophage-like phenotype in the lung premetastatic microenvironment. Therefore, LD-AET can disrupt the lung premetastatic microenvironment, ultimately inhibiting pulmonary metastases.
Figure 1.. Low dose adjuvant epigenetic therapy disrupts the lung premetastatic microenvironment by affecting MDSCs.
a, Timeline of murine metastatic models with treatment schedules. b, Longitudinal H&E and immunofluorescence staining of lung tissue showed the presence of tumour cells from day 6 in the LLC metastasis model. Immunofluorescence staining was performed using GFP (green) antibodies. Merged images contain DAPI DNA staining demarcating cell nuclei (blue). Scale bar, 2 mm. Graph shows the area and numbers of metastatic nodules (n=3 mice at each time point). Two-sample two-sided t-test. c, Immune cell profiles of lungs in the LLC metastasis model. Single cell suspensions from both lungs were analyzed by FACS (n=3 mice at each timepoint). NC: negative control, normal lung from C57BL/6 mice. Two-sample two-sided t-test was used in comparison with NC. d, FACS showing lung MDSCs in LLC metastasis mice at day 3 were depleted using Pep-H6 (left panels). Column diagram showing the effect of Pep-H6 on the percentages of lung MDSCs and macrophages (middle panels) at day 3 (n=3 mice in each group). Two-sample two-sided t-test. Kaplan-Meier curves showing disease-free and overall survival of LLC metastasis mice after MDSCs depletion (right panels). Two-sided log-rank test. Pep-irrel, irrelevant control peptibody. e, Kaplan-Meier curves showing disease-free and overall survival of LLC metastasis mice after transfusion of lung MDSCs (5 × 106, left panels) and of bone marrow M-MDSCs (5 × 106, right panels) or bone marrow PMN-MDSCs (5 × 106, right panels). All transfusions conducted on day 1 and day 4, respectively. Two-sided log-rank test. f, FACS showing representative effects of low dose adjuvant epigenetic therapy (LD-AET) on lung MDSCs in LLC and HNM007 metastasis mice at day 3. Column diagrams showing the effect of LD-AET on lung MDSCs in LLC and HNM007 mice (n=3 at each timepoint). Two-sample two-sided t-test. Bars show mean ± s.e.m. *P< 0.05, **P< 0.01, ***P< 0.001.
Figure 2.. Low dose adjuvant epigenetic therapy inhibits M-MDSCs migration from the bone marrow to the lung premetastatic microenvironment by downregulating CCR2 expression.
The effect of LD-AET on M-MDSCs transferred from CD45.1 to CD45.2 mice (a) and the trafficking ability of adoptively transferred M-MDSCs from LD-AET or vehicle treated CD45.1 mice in CD45.2 recipient mice (b). FACS and graphs showing percentages and absolute numbers of donor-derived cell subsets (CD45.1+ cells) in the both lungs of LLC recipient metastasis mice (n=3 mice per group). c, Cell sorting schema for harvesting M-MDSCs on day 3 post-resection (left panels). Volcano plots showing RNA expression differences of bone marrow and lung M-MDSCs between LD-AET and vehicle treated LLC metastasis mice (right panels, n=3 biological replicates). d, Agilent cDNA array expression of chemokines/chemokine receptors in M-MDSCs from bone marrow and lungs of LD-AET and vehicle treated LLC metastasis mice (n=3 biological replicates). FDR adjusted P values of CCR2 are 0.005 (bone marrow) and 0.016 (lung). e, Effect of LD-AET on CCR2 expression in bone marrow M-MDSCs (BM-M-MDSCs) from LLC metastasis mice on day 3 by quantitative-PCR (left panel, n=3 biological replicates) and FACS (right panel). f, Transwell migration assay of sorted BM-M-MDSCs from LD-AET or vehicle treated LLC metastasis mice (day 3) induced by CCL2 for 60 minutes. Fold changes normalized to migration of the cells in the unstimulated mock group (set at 1) (n=3 biological replicates). g, FACS showing representative results of lung MDSCs in CCR2 KO and wild-type (WT) LLC and HNM007 (left panels) metastasis mice at day 3. Kaplan-Meier curves showing disease-free and overall survival of CCR2 KO and WT LLC (upper panels) and HNM007 (lower panels) metastasis mice. Two-sided log-rank test. a-f, two-sample two-sided t-test. Bars show mean ± s.e.m.
Figure 3.. Low dose adjuvant epigenetic therapy skews M-MDSCs towards an interstitial macrophage-like population in the lung premetastatic microenvironment.
a, GSEA analysis revealed that macrophage or myeloid differentiation and activation gene sets were upregulated (left panel) in lung M-MDSCs from LLC metastasis mice treated with LD-AET. Representative upregulated GSEA plots with core-enriched genes (right panels). NES: normalized enrichment score. FDR: false discovery rate. Colour gradation is representative of log2 fold change over mock (n=3 biological replicates). Gene set enrichment P values, NES values and FDR values reported are calculated with 1,000 permutations in the GSEA software. FDR q-value < 0.25 was deemed significant. b, Significant changes of representative transcription factors (FDR adjusted _P_-value<0.05) associated with monocytic differentiation (n=3 biological replicates). c, FACS showing the differentiation of sorted M-MDSCs in vitro. Splenic M-MDSCs from LLC metastasis mice were cultured for 3 days with tumour conditioned medium (TCM) (n=3 biological replicates). d, The top 200 significantly upregulated genes (FDR adjusted _P_-value<0.05) were mapped back to the reference ImmGen populations, the following populations from naïve mice were used: Lung CD103+ dendritic cells, DC_103+11b−_Lu; CD11b+24+lung dendritic cells, DC_103–11b+24+_Lu; Lung interstitial macrophages, MF_11c-11b+_Lu; Lung alveolar macrophages, MF_Alv_Lu; Lung monocytes, Mo_Lu. The expressions of these 200 genes in six populations were transformed by zero-mean normalization. Mann-Whitney U test, two-sided. n=3 biological replicates. e, FACS showing LD-AET can skew the differentiation of transferred M-MDSCs towards an interstitial macrophage-like phenotype in vivo. Purified 5×106 CD45.1+ BM-M-MDSCs (day0) were adoptively transferred into CD45.2+ recipient mice within 24 hours post-resection (n=3 in each group) in LLC metastasis model. Mice received LD-AET or vehicle for 36 hours. b, c and e, two-sample two-sided t-test. All bars show mean ± s.e.m.
Figure 4.. Low dose adjuvant epigenetic therapy inhibits pulmonary metastases and prolongs overall survival in murine models mainly by affecting MDSCs.
a, Representative H&E-stained images of lung sections from LLC metastasis mice treated with LD-AET or vehicle at different time points after surgery. Scale bar, 2 mm. Graph shows the area and numbers of metastatic nodules. At each time point, 3 mice were sacrificed for analysis. For each sample, sections from 3 levels were analyzed. Tumour area was quantitated using Aperio Imagescope software. Two-sample two-sided t-test. All bars are mean ± s.e.m. b, Kaplan-Meier curves showing the disease-free and overall survival of LLC metastasis mice treated with LD-AET or vehicle. c. Kaplan-Meier curves showing the disease-free and overall survival of the LLC metastasis mice treated with paclitaxel plus cisplatin chemotherapy, LD-AET or vehicle. d, Kaplan-Meier curves showing the disease-free survival and overall survival of mice treated with vehicle, IgG (isotype control), anti-CD4/CD8 (T-cell depletion antibody), LD-AET, and anti-CD4/CD8 combination with LD-AET in LLC metastasis model. e, Kaplan-Meier curves showing the disease-free survival and overall survival of mice treated with vehicle, CCR2 antagonist (RS504393, Sigma), LD-AET and RS504393 in combination with LD-AET in the LLC metastasis model. b-e, two-sided log-rank test. *P < 0.05, **P < 0.01, ***P < 0.001.
Comment in
- Epigenetic Therapy Can Suppress Premetastatic Changes in the Lung.
[No authors listed] [No authors listed] Cancer Discov. 2020 May;10(5):OF10. doi: 10.1158/2159-8290.CD-RW2020-036. Epub 2020 Mar 6. Cancer Discov. 2020. PMID: 32144095 - Tweaking the DNA of myeloid cells curbs cancer spread.
Ghasemi A, De Palma M. Ghasemi A, et al. Nature. 2020 Mar;579(7798):196-197. doi: 10.1038/d41586-020-00481-y. Nature. 2020. PMID: 32152601 No abstract available.
Similar articles
- Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1β-mediated increase in E-selectin expression.
Shi H, Zhang J, Han X, Li H, Xie M, Sun Y, Liu W, Ba X, Zeng X. Shi H, et al. Int J Cancer. 2017 Mar 15;140(6):1370-1383. doi: 10.1002/ijc.30538. Int J Cancer. 2017. PMID: 27885671 - CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer.
Wang D, Sun H, Wei J, Cen B, DuBois RN. Wang D, et al. Cancer Res. 2017 Jul 1;77(13):3655-3665. doi: 10.1158/0008-5472.CAN-16-3199. Epub 2017 Apr 28. Cancer Res. 2017. PMID: 28455419 Free PMC article. - Targeting Myeloid-Derived Suppressor Cells for Premetastatic Niche Disruption After Tumor Resection.
Tang F, Tie Y, Hong W, Wei Y, Tu C, Wei X. Tang F, et al. Ann Surg Oncol. 2021 Jul;28(7):4030-4048. doi: 10.1245/s10434-020-09371-z. Epub 2020 Nov 30. Ann Surg Oncol. 2021. PMID: 33258011 Free PMC article. Review. - Targeted Deletion of CXCR2 in Myeloid Cells Alters the Tumor Immune Environment to Improve Antitumor Immunity.
Yang J, Yan C, Vilgelm AE, Chen SC, Ayers GD, Johnson CA, Richmond A. Yang J, et al. Cancer Immunol Res. 2021 Feb;9(2):200-213. doi: 10.1158/2326-6066.CIR-20-0312. Epub 2020 Nov 11. Cancer Immunol Res. 2021. PMID: 33177110 Free PMC article. - Myeloid-derived suppressor cells: Important contributors to tumor progression and metastasis.
Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Safarzadeh E, et al. J Cell Physiol. 2018 Apr;233(4):3024-3036. doi: 10.1002/jcp.26075. Epub 2017 Aug 3. J Cell Physiol. 2018. PMID: 28661031 Review.
Cited by
- Epigenetic modifications in the accumulation and function of myeloid-derived suppressor cells.
Xu L, Zhou C, Liang Y, Fan T, Zhang F, Chen X, Yuan W. Xu L, et al. Front Immunol. 2022 Nov 11;13:1016870. doi: 10.3389/fimmu.2022.1016870. eCollection 2022. Front Immunol. 2022. PMID: 36439186 Free PMC article. Review. - Epigenetic Mechanisms beyond Tumour-Stroma Crosstalk.
Gagliano T, Brancolini C. Gagliano T, et al. Cancers (Basel). 2021 Feb 22;13(4):914. doi: 10.3390/cancers13040914. Cancers (Basel). 2021. PMID: 33671588 Free PMC article. Review. - Distinct Cell Adhesion Signature Defines Glioblastoma Myeloid-Derived Suppressor Cell Subsets.
Bayik D, Bartels CF, Lovrenert K, Watson DC, Zhang D, Kay K, Lee J, Lauko A, Johnson S, Lo A, Silver DJ, McGraw M, Grabowski M, Mohammadi AM, Veglia F, Fan Y, Vogelbaum MA, Scacheri P, Lathia JD. Bayik D, et al. Cancer Res. 2022 Nov 15;82(22):4274-4287. doi: 10.1158/0008-5472.CAN-21-3840. Cancer Res. 2022. PMID: 36126163 Free PMC article. - An NR2F1-specific agonist suppresses metastasis by inducing cancer cell dormancy.
Khalil BD, Sanchez R, Rahman T, Rodriguez-Tirado C, Moritsch S, Martinez AR, Miles B, Farias E, Mezei M, Nobre AR, Singh D, Kale N, Sproll KC, Sosa MS, Aguirre-Ghiso JA. Khalil BD, et al. J Exp Med. 2022 Jan 3;219(1):e20210836. doi: 10.1084/jem.20210836. Epub 2021 Nov 23. J Exp Med. 2022. PMID: 34812843 Free PMC article. - Surgical Treatment of Osteosarcoma Induced Distant Pre-Metastatic Niche in Lung to Facilitate the Colonization of Circulating Tumor Cells.
Tang F, Tie Y, Lan TX, Yang JY, Hong WQ, Chen SY, Shi HH, Li LQ, Zeng H, Min L, Wei YQ, Tu CQ, Wei XW. Tang F, et al. Adv Sci (Weinh). 2023 Oct;10(28):e2207518. doi: 10.1002/advs.202207518. Epub 2023 Aug 16. Adv Sci (Weinh). 2023. PMID: 37585564 Free PMC article.
References
- Arriagada R et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J. Clin. Oncol. 28, 35–42 (2010). - PubMed
- Bonapace L et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515, 130–133 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases