Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer's Disease Progression - PubMed (original) (raw)
Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer's Disease Progression
Laura Gil et al. Int J Mol Sci. 2020.
Abstract
Background: Recent reports point to a nuclear origin of Alzheimer's disease (AD). Aged postmitotic neurons try to repair their damaged DNA by entering the cell cycle. This aberrant cell cycle re-entry involves chromatin modifications where nuclear Tau and the nuclear lamin are involved. The purpose of this work was to elucidate their participation in the nuclear pathological transformation of neurons at early AD.
Methodology: The study was performed in hippocampal paraffin embedded sections of adult, senile, and AD brains at I-VI Braak stages. We analyzed phospho-Tau, lamins A, B1, B2, and C, nucleophosmin (B23) and the epigenetic marker H4K20me3 by immunohistochemistry.
Results: Two neuronal populations were found across AD stages, one is characterized by a significant increase of Lamin A expression, reinforced perinuclear Lamin B2, elevated expression of H4K20me3 and nuclear Tau loss, while neurons with nucleoplasmic Lamin B2 constitute a second population.
Conclusions: The abnormal cell cycle reentry in early AD implies a fundamental neuronal transformation. This implies the reorganization of the nucleo-cytoskeleton through the expression of the highly regulated Lamin A, heterochromatin repression and building of toxic neuronal tangles. This work demonstrates that nuclear Tau and lamin modifications in hippocampal neurons are crucial events in age-related neurodegeneration.
Keywords: Alzheimer’s disease; Lamin A; Lamin B2; Tau protein; cell-cycle; heterochromatin; hippocampus; neurofibrillary tangles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
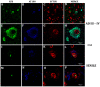
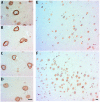
Neurons containing phosphorylated Tau in the CA1 and CA3 hippocampal regions in mature, elderly, and AD I-VI stages of Braak. Immunopositive AT100 nuclei in mature and senile neurons (A,B,K,L). AD I-II nuclei present both intense and fading immunostaining (C,M), while in middle AD stages it shows cytoplasmic and only some slightly positive nuclei (D,N). Exclusive localization in the cytoplasm in late AD (E,O). AT8 is present in nuclei of senile and early AD (G,H,Q,R) but not in mature stages (F,P). Tangles and immunopositive neuropile in the middle and late AD stages (I,J,S,T). scale bar10 µm.
Figure 2
Confocal analysis of nuclear and cytoplasmic phosphorylated Tau in senile CA1 hippocampal neurons and at AD III-IV Braak stages**.** Colocalization of two phosphorylation sites (S212-T214) and (S202-T205) was tested with AT100 and a rabbit monoclonal antibody (AH36), which recognizes the same site as AT8 antibody. The term AT8 was kept in order to follow in line with the previous figure. View of CA1 region at AD III-IV showing the characteristic tauopathy through AT100 (blue), AT8 (green) and nuclei stained in red with SYTOX (A–D). Detail of a neurofibrillary tangle with cytoplasmic AT100 (E) and AT8 (F). Nuclear presence of the site recognized by AT8 (I,M), and of AT100 site (J) in a neuron from senile hippocampus. AT100 was also identified in the cytoplasm of senile neurons (N,P). Senile CA1 neuronal nuclei can simultaneously express AT8 and AT100 (L), or nuclear AT8 and cytoplasmic AT100 (P).
Figure 3
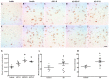
Presence of Lamin A immunopositive pyramidal neurons in CA1 and CA3. Neurons from adult (A,F) and elderly (B,G) subjects lack immunopositivity to Lamin A. Increased membrane expression of Lamin A characterizes early AD stages (C,H) and the presence of immunopositivity continues at late AD stages (D,E,I,J), 40X microphotographs, scale bar—10 µm. Quantification of immunopositivity. Significant increases of Lamin A in total intensity (K), percentage of positive cells (L), and mean intensity (M). Each point represents the image analysis of 50 cells per subject in CA1 and CA3 regions. Graphs express mean ± SD, * p < 0.05. See the text for further details of image analysis and statistics.
Figure 4
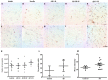
Lamin B2 in pyramidal hippocampal neurons. Lamin B2 immunopositivity in adults is mostly perinuclear (A,F) and starts appearing in the nucleoplasm in senile subjects (B,G). Pyramidal neurons in both CA1 and CA3 present either intense perinuclear immunopositivity or homogeneous staining over the nucleoplasm (C–E,H–J), 40× microphotographs, scale bar—10 µm. Quantification of immunopositivity. Lamin B2 immunostaining in nucleoplasm increased significantly in the middle and late AD stages (K,M). The intensity of the membranes clearly reveals two populations of neurons (H, high and low intensity), and Lamin B2 mean intensity increased significantly in AD (L). Each point represents the image analysis of 50 cells per subject in CA1 and CA3 regions. Graphs express mean ± SD, * p < 0.05. See the text for further details of image analysis and statistics.
Figure 5
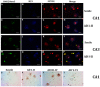
Lamin B1 in pyramidal hippocampal neurons. Low-intensity Lamin B1 immunopositivity is present at all conditions studied (A–J), with slightly higher levels at AD V-VI (E–J). Low-intensity Lamin B1 immunopositivity is present at all conditions studied (A–J), with slightly higher levels at AD V-VI, oligodendrocytes and microglia intensely stained (E,J). Lamin B1 did not present quantitative changes across the different conditions (K-M). Each point represents the image analysis of 50 cells per subject in CA1 and CA3 regions. Graphs express mean ± SD, * p < 0.05. See the text for further details of image analysis and statistics.
Figure 6
Confocal analysis of H4K20me3 (green), and nucleoli immunofluorescence through nucleophosmin antibody (B23, blue) and nucleic acids through SYTOX (red). Neurons from a senile subject present in CA1 (A) and CA3 (I) scarce positive green marks, and well-delimited nucleoli (B,J), and the epigenetic H4K20me3 marks localized in the nucleolar chromatin (C,D,K,L). A marked increase of nuclear speckles and spots around the nucleoli and adjacent to the nuclear lamina is observed at AD (I-II) (E,M) and B23 immunofluorescence is not limited to the nucleoli but also dispersed in the cytoplasm (F,N,H,P), and H4K20me3 marks are not only in the nucleolar chromatin (G,O) but also adjacent to the nuclear lamina (H,P). H4K20me3 immunostaining. Distribution of H4K20me3 immunopositivity around the nucleolus (NADs) and adjacent to the nuclear lamina (LADs) (Q). Intensely marked nuclei at AD I-II stages (R) and AD II-IV stages (S) and null to slight positivity at late AD stages (T,L), scale bar—10 µm.
Figure 7
Relationship of Lamin B2 with Lamin A at early AD stages. Lamin B2 immunopositivity (DAB-brown) covers the nucleoplasm of neurons with nucleoli displaced to the periphery or without nucleoli and signs of degeneration (A,D). A reinforced Lamin B2 associated with Lamin A (red- amino-ethyl-carbazole) characterizes neurons with euchromatic nuclei with prominent nucleoli (B,C). In the hippocampus at AD I-II stages the two neuron populations coexist in CA1 (E) and CA3 (F), scale bar—5 µm.
Figure 8
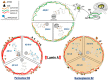
Nuclear dynamics of Tau, nuclear lamins, and H4K20me3 in the hippocampus of adult, senile AD I-II, AD III-IV, and AD V-VI Braak stages. Adult neurons have low phosphorylated Tau-AT100 expression and Lamin B1 and Lamin B2 are the components of NL. Senile neuron increment their content of phosphorylated Tau-AT100 and AT8. Changes in Lamin B2 determine the two types of neurons, those with reinforced perinuclear Lamin B2 (left) and those with nucleoplasmic Lamin B2 (right). Lamin A (left) expression is associated with H4K20me3 repressive marks in LADs and NADs. The increased presence of cytoplasmic Tau leads to hyperphosphorylation, oligomer and NFT formation.
Similar articles
- Aging and Alzheimer's disease connection: Nuclear Tau and lamin A.
Gil L, Niño SA, Capdeville G, Jiménez-Capdeville ME. Gil L, et al. Neurosci Lett. 2021 Apr 1;749:135741. doi: 10.1016/j.neulet.2021.135741. Epub 2021 Feb 18. Neurosci Lett. 2021. PMID: 33610669 Review. - Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain.
Takamori Y, Tamura Y, Kataoka Y, Cui Y, Seo S, Kanazawa T, Kurokawa K, Yamada H. Takamori Y, et al. Eur J Neurosci. 2007 Mar;25(6):1653-62. doi: 10.1111/j.1460-9568.2007.05450.x. Eur J Neurosci. 2007. PMID: 17432957 - LINCing lamin B2 to neuronal migration: growing evidence for cell-specific roles of B-type lamins.
Coffinier C, Fong LG, Young SG. Coffinier C, et al. Nucleus. 2010 Sep-Oct;1(5):407-11. doi: 10.4161/nucl.1.5.12830. Nucleus. 2010. PMID: 21278813 Free PMC article. - Nuclear envelope dispersion triggered by deregulated Cdk5 precedes neuronal death.
Chang KH, Multani PS, Sun KH, Vincent F, de Pablo Y, Ghosh S, Gupta R, Lee HP, Lee HG, Smith MA, Shah K. Chang KH, et al. Mol Biol Cell. 2011 May;22(9):1452-62. doi: 10.1091/mbc.E10-07-0654. Epub 2011 Mar 9. Mol Biol Cell. 2011. PMID: 21389115 Free PMC article. - Alzheimer's disease: An acquired neurodegenerative laminopathy.
Frost B. Frost B. Nucleus. 2016 May 3;7(3):275-83. doi: 10.1080/19491034.2016.1183859. Epub 2016 May 11. Nucleus. 2016. PMID: 27167528 Free PMC article. Review.
Cited by
- Biochemical Pathways of Cellular Mechanosensing/Mechanotransduction and Their Role in Neurodegenerative Diseases Pathogenesis.
Tortorella I, Argentati C, Emiliani C, Morena F, Martino S. Tortorella I, et al. Cells. 2022 Oct 1;11(19):3093. doi: 10.3390/cells11193093. Cells. 2022. PMID: 36231055 Free PMC article. Review. - Importin-Mediated Pathological Tau Nuclear Translocation Causes Disruption of the Nuclear Lamina, TDP-43 Mislocalization and Cell Death.
Candia RF, Cohen LS, Morozova V, Corbo C, Alonso AD. Candia RF, et al. Front Mol Neurosci. 2022 May 3;15:888420. doi: 10.3389/fnmol.2022.888420. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35592115 Free PMC article. - Nuclear face of Tau: an inside player in neurodegeneration.
Younas N, Saleem T, Younas A, Zerr I. Younas N, et al. Acta Neuropathol Commun. 2023 Dec 12;11(1):196. doi: 10.1186/s40478-023-01702-x. Acta Neuropathol Commun. 2023. PMID: 38087392 Free PMC article. Review. - Development of an accelerated cellular model for early changes in Alzheimer's disease.
Xue H, Gate S 3rd, Gentry E, Losert W, Cao K. Xue H, et al. Sci Rep. 2023 Oct 26;13(1):18384. doi: 10.1038/s41598-023-45826-5. Sci Rep. 2023. PMID: 37884611 Free PMC article. - Unravelling the mechanotransduction pathways in Alzheimer's disease.
Donnaloja F, Limonta E, Mancosu C, Morandi F, Boeri L, Albani D, Raimondi MT. Donnaloja F, et al. J Biol Eng. 2023 Mar 28;17(1):22. doi: 10.1186/s13036-023-00336-w. J Biol Eng. 2023. PMID: 36978103 Free PMC article. Review.
References
- Shumaker D.K., Dechat T., Kohlmaier A., Adam S.A., Bozovsky M.R., Erdos M.R., Eriksson M., Goldman A.E., Khuon S., Collins F.S., et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. USA. 2006;103:8703–8708. doi: 10.1073/pnas.0602569103. - DOI - PMC - PubMed
- Hozák P., Sasseville A.M., Raymond Y., Cook P.R. Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in human cells. Pt 2J. Cell Sci. 1995;108:635–644. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical